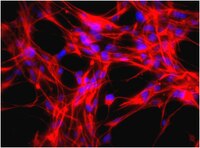
Differentiation of non-mesencephalic neural stem cells towards dopaminergic neurons.
Rössler, R, et al.
Neuroscience, 170: 417-28 (2010)
2009
Kivonat megmutatása
Neural stem cells (NSCs), either isolated from fetal or adult human brain or derived from induced pluripotent stem cells, are now considered major candidates for in vitro generation of transplantable dopaminergic (DA) neurons and modeling of Parkinson's disease. It is generally thought that in vitro differentiation of neural stem cells into meso-diencephalic dopaminergic neurons, requires recapitulation of dopaminergic differentiation pathway normally occurring in the ventral mesencephalon during embryogenesis. This dopaminergic pathway is partially activated by a combination of the extracellular induction factors Sonic Hedgehog (Shh), Fibroblast Growth Factor 8 (FGF8) and Wnt1 that trigger specific intracellular transcription cascades. In vitro mimicking of these embryonic ventral mesencephalic conditions has been successful for dopaminergic differentiation of embryonic stem cells and ventral mesencephalic NSCs. Dopaminergic differentiation of non-mesencephalic NSCs (nmNSCs), however, is considered arduous. Here we examine whether Shh, FGF8 and Wnt1 can activate typical dopaminergic transcription factors, such as Lmx1a, Msx1 and Otx2 in nmNSCs. We found that Shh, FGF8 and Wnt1 induced the expression of Lmx1a and Otx2 in nmNSCs resulting in the differentiation of up to 39% of the nmNSCs into neurons expressing Pitx3. However, only a low number ( approximately 13%) of these cells became more DA-like neurons also expressing tyrosine hydroxylase (TH). The histone deacetylase (HDAC)-inhibitor trichostatin A combined with Shh, FGF8 and Wnt1 caused orchestrated induction of Lmx1a, Otx2, Msx1 plus the early DA transcription factor En1. Now significantly increased numbers of TH ( approximately 22%) and Pitx3 ( approximately 33%) neurons were observed. Most of these cells coexpressed the DA markers DAT and Vmat2. Taken together, we demonstrate that nmNSCs indeed can be differentiated towards DA-like neurons, but this differentiation is far from complete in comparison to ventral mesencephalic NSCs and embryonic stem cells; most likely, the nmNSCs lack the proper "primed" epigenetic state of these cells for DA differentiation facilitating the induction of DA specific transcription factors. | 20643196
 |
Absence of annulus in human asthenozoospermia: case report.
Lhuillier, P, et al.
Hum. Reprod., 24: 1296-303 (2009)
2009
Kivonat megmutatása
The annulus is a septin-based ring structure located at the junction of the midpiece (MP) and the principal piece (PP) of spermatozoa flagellum. In the mouse, deletion of Septin 4, a structural component of the sperm annulus, prevents annulus formation and leads to MP-PP disjunction, flagellar bending, asthenozoospermia and male sterility. Testis anion transporter 1 (Tat1) is a germ cell-specific member of the SLC26 anion transporter family and is co-expressed with Septin 4 at the sperm annulus. Interestingly, Tat1 null sperm bear an atrophic annulus, causing a phenotype similar to that of Sept4 null sperm. We searched for Tat1 misexpression and/or mislocalization in spermatozoa from asthenozoospermic subjects (n = 75) and controls by performing an immunofluorescence detection assay on sperm smear preparations. We found one patient showing moderate asthenozoospermia, with 97% of sperm lacking Tat1, Septin 4 and Septin 7 proteins at the annulus. We confirmed the absence of the annulus structure by transmission electron microscopy and observed that spermatozoa from the patient displayed MP-PP disjunction and abnormal mitochondrial organization. We show that the structural defects in sperm are not caused by abnormal transcription or point mutations of the TAT1 and SEPT4 genes; however, although both proteins are expressed, they are not properly localized at sperm annulus. The case we studied, so far unreported in human, confirms the involvement of Tat1 and Septin proteins in the constitution of the annulus, but also raises questions about the function of this structure in human sperm motility. | 19221096
 |
Developmental changes and injury induced disruption of the radial organization of the cortex in the immature rat brain revealed by in vivo diffusion tensor MRI.
Sizonenko, SV; Camm, EJ; Garbow, JR; Maier, SE; Inder, TE; Williams, CE; Neil, JJ; Huppi, PS
Cerebral cortex (New York, N.Y. : 1991)
17
2609-17
2007
Kivonat megmutatása
During brain development, morphological changes modify the cortex from its immature radial organization to its mature laminar appearance. Applying in vivo diffusion tensor imaging (DTI), the microstructural organization of the cortex in the immature rat was analyzed and correlated to neurohistopathology. Significant differences in apparent diffusion coefficient (ADC) and fractional anisotropy (FA) were detected between the external (I-III) and deep (IV-VI) cortical layers in postnatal day 3 (P3) and P6 pups. With cortical maturation, ADC was reduced in both cortical regions, whereas a decrease in FA was only seen in the deep layers. A distinct radial organization of the external cortical layers with the eigenvectors perpendicular to the pial surface was observed at both ages. Histology revealed maturational differences in the cortical architecture with increased neurodendritic density and reduction in the radial glia scaffolding. Early DTI after hypoxia-ischemia at P3 shows reduced ADC and FA in the ipsilateral cortex that persisted at P6. Cortical DTI eigenvector maps reveal microstructural disruption of the radial organization corresponding to regions of neuronal death, radial glial disruption, and astrocytosis. Thus, the combined use of in vivo DTI and histopathology can assist in delineating normal developmental changes and postinjury modifications in the immature rodent brain. | 17259644
 |