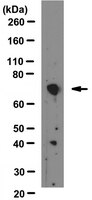
Inhibition of SHP2 in basal-like and triple-negative breast cells induces basal-to-luminal transition, hormone dependency, and sensitivity to anti-hormone treatment.
Zhao, H; Agazie, YM
BMC cancer
15
109
2015
Kivonat megmutatása
The Src homology phosphotyrosyl phosphatase 2 (SHP2) is a positive effector of cell growth and survival signaling as well transformation induced by multiple tyrosine kinase oncogenes. Since the basal-like and triple-negative breast cancer (BTBC) is characterized by dysregulation of multiple tyrosine kinase oncogenes, we wanted to determine the importance of SHP2 in BTBC cell lines.Short hairpin RNA-based and dominant-negative expression-based SHP2 inhibition techniques were used to interrogate the functional importance of SHP2 in BTBC cell biology. In addition, cell viability and proliferation assays were used to determine hormone dependency for growth and sensitivity to anti-estrogen treatment.We show that inhibition of SHP2 in BTBC cells induces luminal-like epithelial morphology while suppressing the mesenchymal and invasive property. We have termed this process as basal-to-luminal transition (BLT). The occurrence of BLT was confirmed by the loss of the basal marker alpha smooth muscle actin and the acquisition of the luminal marker cytokeratin 18 (CK18) expression. Furthermore, the occurrence of BLT led to estrogen receptor alpha (ERα) expression, hormone dependency, and sensitivity to tamoxifen treatment.Our data show that inhibition of SHP2 induces BLT, ERα expression, dependency on estrogen for growth, and sensitivity to anti-hormone therapy. Therefore, inhibition of SHP2 may provide a therapeutic benefit in basal-like and triple-negative breast cancer. | 25885600
 |
Folate deficiency decreases apoptosis of endometrium decidual cells in pregnant mice via the mitochondrial pathway.
Liao, XG; Li, YL; Gao, RF; Geng, YQ; Chen, XM; Liu, XQ; Ding, YB; Mu, XY; Wang, YX; He, JL
Nutrients
7
1916-32
2015
Kivonat megmutatása
It is well known that maternal folate deficiency results in adverse pregnancy outcomes. In addition to aspects in embryonic development, maternal uterine receptivity and the decidualization of stromal cells is also very important for a successful pregnancy. In this study, we focused on endometrium decidualization and investigated whether apoptosis, which is essential for decidualization, was impaired. Flow cytometry and TUNEL detection revealed that apoptosis of mouse endometrium decidual cells was suppressed in the dietary folate-deficient group on Days 7 and 8 of pregnancy (Day 1 = vaginal plug) when decidua regression is initiated. The endometrium decidual tissue of the folate deficiency group expressed less Bax compared to the normal diet group while they had nearly equal expression of Bcl2 protein. Further examination revealed that the mitochondrial transmembrane potential (ΔΨm) decreased, and the fluorescence of diffuse cytoplasmic cytochrome c protein was detected using laser confocal microscopy in normal decidual cells. However, no corresponding changes were observed in the folate-deficient group. Western blotting analyses confirmed that more cytochrome c was released from mitochondria in normal decidual cells. Taken together, these results demonstrated that folate deficiency could inhibit apoptosis of decidual cells via the mitochondrial apoptosis pathway, thereby restraining decidualization of the endometrium and further impairing pregnancy. | 25781218
 |
Masseter muscle myofibrillar protein synthesis and degradation in an experimental critical illness myopathy model.
Akkad, H; Corpeno, R; Larsson, L
PloS one
9
e92622
2014
Kivonat megmutatása
Critical illness myopathy (CIM) is a debilitating common consequence of modern intensive care, characterized by severe muscle wasting, weakness and a decreased myosin/actin (M/A) ratio. Limb/trunk muscles are primarily affected by this myopathy while cranial nerve innervated muscles are spared or less affected, but the mechanisms underlying these muscle-specific differences remain unknown. In this time-resolved study, the cranial nerve innervated masseter muscle was studied in a unique experimental rat intensive care unit (ICU) model, where animals were exposed to sedation, neuromuscular blockade (NMB), mechanical ventilation, and immobilization for durations varying between 6 h and 14d. Gel electrophoresis, immunoblotting, RT-PCR and morphological staining techniques were used to analyze M/A ratios, myofiber size, synthesis and degradation of myofibrillar proteins, and levels of heat shock proteins (HSPs). Results obtained in the masseter muscle were compared with previous observations in experimental and clinical studies of limb muscles. Significant muscle-specific differences were observed, i.e., in the masseter, the decline in M/A ratio and muscle fiber size was small and delayed. Furthermore, transcriptional regulation of myosin and actin synthesis was maintained, and Akt phosphorylation was only briefly reduced. In studied degradation pathways, only mRNA, but not protein levels of MuRF1, atrogin-1 and the autophagy marker LC3b were activated by the ICU condition. The matrix metalloproteinase MMP-2 was inhibited and protective HSPs were up-regulated early. These results confirm that the cranial nerve innervated masticatory muscles is less affected by the ICU-stress response than limb muscles, in accordance with clinical observation in ICU patients with CIM, supporting the model' credibility as a valid CIM model. | 24705179
 |
Endothelial nitric oxide synthase and superoxide mediate hemodynamic initiation of intracranial aneurysms.
Liaw, N; Fox, JM; Siddiqui, AH; Meng, H; Kolega, J
PloS one
9
e101721
2014
Kivonat megmutatása
Hemodynamic insults at arterial bifurcations are believed to play a critical role in initiating intracranial aneurysms. Recent studies in a rabbit model indicate that aneurysmal damage initiates under specific wall shear stress conditions when smooth muscle cells (SMCs) become pro-inflammatory and produce matrix metalloproteinases (MMPs). The mechanisms leading to SMC activation and MMP production during hemodynamic aneurysm initiation are unknown. The goal is to determine if nitric oxide and/or superoxide induce SMC changes, MMP production and aneurysmal remodeling following hemodynamic insult.Bilateral common carotid artery ligation was performed on rabbits (n = 19, plus 5 sham operations) to induce aneurysmal damage at the basilar terminus. Ligated animals were treated with the nitric oxide synthase (NOS) inhibitor LNAME (n = 7) or the superoxide scavenger TEMPOL (n = 5) and compared to untreated animals (n = 7). Aneurysm development was assessed histologically 5 days after ligation. Changes in NOS isoforms, peroxynitrite, reactive oxygen species (ROS), MMP-2, MMP-9, and smooth muscle α-actin were analyzed by immunohistochemistry.LNAME attenuated ligation-induced IEL loss, media thinning and bulge formation. In untreated animals, immunofluorescence showed increased endothelial NOS (eNOS) after ligation, but no change in inducible or neuronal NOS. Furthermore, during aneurysm initiation ROS increased in the media, but not the intima, and there was no change in peroxynitrite. In LNAME-treated animals, ROS production did not change. Together, this suggests that eNOS is important for aneurysm initiation but not by producing superoxide. TEMPOL treatment reduced aneurysm development, indicating that the increased medial superoxide is also necessary for aneurysm initiation. LNAME and TEMPOL treatment in ligated animals restored α-actin and decreased MMPs, suggesting that eNOS and superoxide both lead to SMC de-differentiation and MMP production.Aneurysm-inducing hemodynamics lead to increased eNOS and superoxide, which both affect SMC phenotype, increasing MMP production and aneurysmal damage. | 24992254
 |
Histological, histochemical, and protein changes after induced malocclusion by occlusion alteration of Wistar rats.
Guerra, Cde S; Carla Lara Pereira, Y; Issa, JP; Luiz, KG; Guimarães, EA; Gerlach, RF; Iyomasa, MM
BioMed research international
2014
563463
2014
Kivonat megmutatása
Although disorders of the stomatognathic system are common, the mechanisms involved are unknown. Our objective was to study the changes in the masseter muscles after unilateral exodontia. Molar extraction was performed on Wistar rats (left side), and the animals were sacrificed after either 14 or 26 days. The masseter muscle was processed for histological analysis, conventional and in situ zymography, and immunohistochemistry. The morphological analysis showed unique and specific characteristics for the experimental group. By conventional zymography no significant values of 72 kDa MMP-2 (P less than 0.05) were found in both of the sides of masseter muscle after 14 and 26 days of unilateral extraction. The in situ zymography showed gelatinolytic activity on all deep masseter muscles, with significant increase on the contralateral side after 14 and 26 days (P less than 0.05). The immunohistochemistry demonstrated greater expression of MMP-2 than MMP-9 and MMP-14 in all masseter muscles and there were few differences in the staining of 4 TIMPs. This knowledge about morphology and molecular masticatory muscle remodeling following environmental interventions can be used to develop clinically successful treatments. | 25028660
 |
Differential effects of caveolin-1 and -2 knockdown on aqueous outflow and altered extracellular matrix turnover in caveolin-silenced trabecular meshwork cells.
Aga, M; Bradley, JM; Wanchu, R; Yang, YF; Acott, TS; Keller, KE
Investigative ophthalmology & visual science
55
5497-509
2014
Kivonat megmutatása
A single nucleotide polymorphism (SNP) identified between caveolin-1 (CAV1) and caveolin-2 (CAV2) on chromosome 7 is associated with glaucoma. One function of CAVs is endocytosis and recycling of extracellular matrix (ECM) components. Here, we generated CAV-silencing lentivirus to evaluate the effects on ECM turnover by trabecular meshwork (TM) cells and to measure the effect on outflow facility in anterior segment perfusion culture.Short hairpin CAV1 and CAV2 silencing and control lentivirus were generated, characterized, and applied to anterior segments in perfusion culture. Colocalization of CAVs with various ECM molecules in TM cells was investigated using immunofluorescence and confocal microscopy. Western immunoblotting and fluorogenic-based enzyme activity assays were used to investigate ECM protein levels and degradation, respectively.Endogenous CAVs colocalized with cortactin at podosome- or invadopodia-like structures (PILS), which are areas of focal ECM degradation. In perfusion culture, outflow rates increased significantly in CAV1-silenced anterior segments, whereas outflow significantly decreased in CAV2-silenced anterior segments. Matrix metalloproteinase (MMP)2 and MMP14, and a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS4) colocalized with both CAVs in TM cells. Protein levels and enzyme activities of MMP/ADAMTS4, fibronectin protein levels, actin stress fibers, and α-smooth muscle actin were all increased in CAV-silenced cells.Caveolin-mediated endocytosis is one mechanism by which TM cells can alter the physiological catabolism of ECM in order to change the composition of the outflow channels in the TM to regulate aqueous outflow resistance. Dysregulation of CAV function could contribute to the pathological changes in ECM that are observed in glaucoma. | 25103269
 |
Expression of MMP-2 and MMP-9 in the rat trigeminal ganglion during the development of temporomandibular joint inflammation.
Nascimento, GC; Rizzi, E; Gerlach, RF; Leite-Panissi, CR
Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica ... [et al.]
46
956-967
2013
Kivonat megmutatása
Orofacial pain is a prevalent symptom in modern society. Some musculoskeletal orofacial pain is caused by temporomandibular disorders (TMDs). This condition has a multi-factorial etiology, including emotional factors and alteration of the masticator muscle and temporomandibular joints (TMJs). TMJ inflammation is considered to be a cause of pain in patients with TMD. Extracellular proteolytic enzymes, specifically the matrix metalloproteinases (MMPs), have been shown to modulate inflammation and pain. The purpose of this investigation was to determine whether the expression and level of gelatinolytic activity of MMP-2 and MMP-9 in the trigeminal ganglion are altered during different stages of temporomandibular inflammation, as determined by gelatin zymography. This study also evaluated whether mechanical allodynia and orofacial hyperalgesia, induced by the injection of complete Freund's adjuvant into the TMJ capsule, were altered by an MMP inhibitor (doxycycline, DOX). TMJ inflammation was measured by plasma extravasation in the periarticular tissue (Evans blue test) and infiltration of polymorphonuclear neutrophils into the synovial fluid (myeloperoxidase enzyme quantification). MMP expression in the trigeminal ganglion was shown to vary during the phases of the inflammatory process. MMP-9 regulated the early phase and MMP-2 participated in the late phase of this process. Furthermore, increases in plasma extravasation in periarticular tissue and myeloperoxidase activity in the joint tissue, which occurred throughout the inflammation process, were diminished by treatment with DOX, a nonspecific MMP inhibitor. Additionally, the increases of mechanical allodynia and orofacial hyperalgesia were attenuated by the same treatment. | 24270905
 |
Role of Cox-2 in vascular inflammation: an experimental model of metabolic syndrome.
Renna, NF; Diez, ER; Lembo, C; Miatello, RM
Mediators of inflammation
2013
513251
2013
Kivonat megmutatása
The objective of this work was to demonstrate the role of COX-2 enzyme at the vascular in experimental model of metabolic syndrome. SHR male WKY rats were employed; they were distributed in 8 groups (n = 8 each): control (W); W + L: WKY rats receiving 20 mg/kg of lumiracoxib by intraesophageal administration; SHR; SHR + L: SHR + 20 mg/kg of lumiracoxib by intraesophageal administration; Fructose-Fed Rats (FFR): WKY rats receiving 10% (w/v) fructose solution in drinking water during all 12 weeks; FFR + L: FFR + 20 mg/kg of lumiracoxib by intraesophageal administration; Fructose-Fed Hypertensive Rats (FFHR): SHR receiving 10% (w/v) fructose solution in drinking water during all 12 weeks; and FFHR + L: FFHR + 20 mg/kg of lumiracoxib by intraesophageal administration. Metabolic variables, blood pressure, morphometric variables, and oxidative stress variables were evaluated; also MMP-2 and MMP-9 (collagenases), VCAM-1, and NF- κ B by Westernblot or IFI were evaluated. FFHR presented all variables of metabolic syndrome; there was also an increase in oxidative stress variables; vascular remodeling and left ventricular hypertrophy were evidenced along with a significant increase in the expression of the mentioned proinflammatory molecules and increased activity and expression of collagenase. Lumiracoxib was able to reverse vascular remodeling changes and inflammation, demonstrating the involvement of COX-2 in the pathophysiology of vascular remodeling in this experimental model. | 23476105
 |
Expression of soluble and functional full-length human matrix metalloproteinase-2 in Escherichia coli.
Gonçalves, AN; Meschiari, CA; Stetler-Stevenson, WG; Nonato, MC; Alves, CP; Espreafico, EM; Gerlach, RF
Journal of biotechnology
157
20-4
2011
Kivonat megmutatása
Characterization of the matrix metalloproteinase-2 (MMP-2) substrates and understanding of its function remain difficult because up to date preparations containing minor amounts of other eukaryotic proteins that are co-purified with MMP-2 are still used. In this work, the expression of a soluble and functional full-length recombinant human MMP-2 (rhMMP-2) in the cytoplasm of Escherichia coli is reported, and the purification of this metalloproteinase is described. Culture of this bacterium at 18°C culminated in maintenance of the soluble and functional rhMMP-2 in the soluble fraction of the E. coli lysate and its purification by affinity with gelatin-sepharose yielded approximately 0.12mg/L of medium. Western Blotting and zymographic analysis revealed that the most abundant form was the 72-kDa MMP-2, but some gelatinolytic bands corresponding to proteins with lower molecular weight were also detected. The obtained rhMMP-2 was demonstrated to be functional in a gelatinolytic fluorimetric assay, suggesting that the purified rhMMP-2 was correctly folded. The method described here involves fewer steps, is less expensive, and is less prone to contamination with other proteinases and MMP inhibitors as compared to expression of rhMMP-2 in eukaryotic tissue culture. This protocol will facilitate the use of the full-length rhMMP-2 expressed in bacteria and will certainly help researchers to acquire new knowledge about the substrates and biological activities of this important proteinase. | 22001844
 |
HPV16 oncoproteins induce MMPs/RECK-TIMP-2 imbalance in primary keratinocytes: possible implications in cervical carcinogenesis.
Cardeal, LB; Boccardo, E; Termini, L; Rabachini, T; Andreoli, MA; di Loreto, C; Longatto Filho, A; Villa, LL; Maria-Engler, SS
PloS one
7
e33585
2011
Kivonat megmutatása
Cervical cancer is the third most common cancer in women worldwide. Persistent infection with high-risk HPV types, principally HPV16 and 18 is the main risk factor for the development of this malignancy. However, the onset of invasive tumor occurs many years after initial exposure in a minority of infected women. This suggests that other factors beyond viral infection are necessary for tumor establishment and progression. Tumor progression is characterized by an increase in secretion and activation of matrix metalloproteinases (MMPs) produced by either the tumor cells themselves or tumor-associated fibroblasts or macrophages. Increased MMPs expression, including MMP-2, MMP-9 and MT1-MMP, has been observed during cervical carcinoma progression. These proteins have been associated with degradation of ECM components, tumor invasion, metastasis and recurrence. However, few studies have evaluated the interplay between HPV infection and the expression and activity of MMPs and their regulators in cervical cancer. We analyzed the effect of HPV16 oncoproteins on the expression and activity of MMP-2, MMP-9, MT1-MMP, and their inhibitors TIMP-2 and RECK in cultures of human keratinocytes. We observed that E7 expression is associated with increased pro-MMP-9 activity in the epithelial component of organotypic cultures, while E6 and E7 oncoproteins co-expression down-regulates RECK and TIMP-2 levels in organotypic and monolayers cultures. Finally, a study conducted in human cervical tissues showed a decrease in RECK expression levels in precancer and cancer lesions. Our results indicate that HPV oncoproteins promote MMPs/RECK-TIMP-2 imbalance which may be involved in HPV-associated lesions outcome. | 22438955
 |