Nuclear localization of the G protein beta 5/R7-regulator of G protein signaling protein complex is dependent on R7 binding protein.
Panicker, LM; Zhang, JH; Posokhova, E; Gastinger, MJ; Martemyanov, KA; Simonds, WF
Journal of neurochemistry
113
1101-12
2009
Kivonat megmutatása
The neuronally expressed G beta(5) subunit is the most structurally divergent among heterotrimeric G beta isoforms and unique in its ability to heterodimerize with the R7 subfamily of regulator of G protein signaling (RGS) proteins. The complex between G beta(5) and R7-type RGS proteins targets the cell nucleus by an unknown mechanism. Although the nuclear targeting of the G beta(5)/R7-RGS complex is proposed to involve the binding of R7-binding protein (R7BP), this theory is challenged by the observations that endogenous R7BP is palmitoylated, co-localizes strongly with the plasma membrane, and has never been identified in the cytosol or nucleus of native neurons or untreated cultured cells. We show here mutant RGS7 lacking the N-terminal Disheveled, EGL-10, Pleckstrin homology domain is expressed in transfected cells but, unlike wild-type RGS7, is excluded from the cell nucleus. As the Disheveled, EGL-10, Pleckstrin homology domain is essential for R7BP binding to RGS7, we studied the subcellular localization of G beta(5) in primary neurons and brain from mice deficient in R7BP. The level of endogenous nuclear G beta(5) and RGS7 in neurons and brains from R7BP knockout mice is reduced by 50-70%. These results suggest that R7BP contributes significantly to the nuclear localization of endogenous G beta(5)/R7-RGS complex in brain. | 20100282
 |
Nuclear localization of G protein beta 5 and regulator of G protein signaling 7 in neurons and brain.
Zhang, JH; Barr, VA; Mo, Y; Rojkova, AM; Liu, S; Simonds, WF
The Journal of biological chemistry
276
10284-9
2001
Kivonat megmutatása
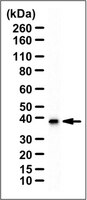
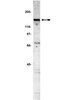
The role that Gbeta(5) regulator of G protein signaling (RGS) complexes play in signal transduction in brain remains unknown. The subcellular localization of Gbeta(5) and RGS7 was examined in rat PC12 pheochromocytoma cells and mouse brain. Both nuclear and cytosolic localization of Gbeta(5) and RGS7 was evident in PC12 cells by immunocytochemical staining. Subcellular fractionation of PC12 cells demonstrated Gbeta(5) immunoreactivity in the membrane, cytosolic, and nuclear fractions. Analysis by limited proteolysis confirmed the identity of Gbeta(5) in the nuclear fraction. Subcellular fractionation of mouse brain demonstrated Gbeta(5) and RGS7 but not Ggamma(2/3) immunoreactivity in the nuclear fraction. RGS7 and Gbeta(5) were tightly complexed in the brain nuclear extract as evidenced by their coimmunoprecipitation with anti-RGS7 antibodies. Chimeric protein constructs containing green fluorescent protein fused to wild-type Gbeta(5) but not green fluorescent fusion proteins with Gbeta(1) or a mutant Gbeta(5) impaired in its ability to bind to RGS7 demonstrated nuclear localization in transfected PC12 cells. These findings suggest that Gbeta(5) undergoes nuclear translocation in neurons via an RGS-dependent mechanism. The novel intracellular distribution of Gbeta(5).RGS protein complexes suggests a potential role in neurons communicating between classical heterotrimeric G protein subunits and/or their effectors at the plasma membrane and the cell nucleus. | 11152459
 |
Selective activation of effector pathways by brain-specific G protein beta5.
Zhang, S; Coso, OA; Lee, C; Gutkind, JS; Simonds, WF
The Journal of biological chemistry
271
33575-9
1996
Kivonat megmutatása
While multiple G protein beta and gamma subunit isoforms have been identified, the implications of this potential diversity of betagamma heterodimers for signaling through betagamma-regulated effector pathways remains unclear. Furthermore the molecular mechanism(s) by which the betagamma complex modulates diverse mammalian effector molecules is unknown. Effector signaling by the structurally distinct brain-specific beta5 subunit was assessed by transient cotransfection with gamma2 in COS cells and compared with beta1. Transfection of either beta1 or beta5 with gamma2 stimulated the activity of cotransfected phospholipase C-beta2 (PLC-beta2), as previously reported. In contrast, cotransfection of beta1 but not beta5 with gamma2 stimulated the mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) pathways even though the expression of beta5 in COS cells was evident by immunoblotting. The G protein beta5 expressed in transfected COS cells was properly folded as its pattern of stable C-terminal proteolytic fragments was identical to that of native brain beta5. The inability of beta5 to activate the MAPK and JNK pathways was not overcome by cotransfection with three additional Ggamma isoforms. These results suggest it is the Gbeta subunit which determines the pattern of downstream signaling by the betagamma complex and imply that the structural features of the betagamma complex mediating effector regulation may differ among effectors. | 8969224
 |