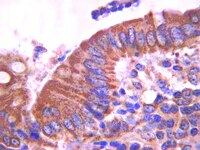
TRPM8 on mucosal sensory nerves regulates colitogenic responses by innate immune cells via CGRP.
de Jong, PR; Takahashi, N; Peiris, M; Bertin, S; Lee, J; Gareau, MG; Paniagua, A; Harris, AR; Herdman, DS; Corr, M; Blackshaw, LA; Raz, E
Mucosal immunology
8
491-504
2015
Kivonat megmutatása
TRPM8 is the molecular sensor for cold; however, the physiological role of TRPM8+ neurons at mucosal surfaces is unclear. Here we evaluated the distribution and peptidergic properties of TRPM8+ fibers in naive and inflamed colons, as well as their role in mucosal inflammation. We found that Trpm8(-/-) mice were hypersusceptible to dextran sodium sulfate (DSS)-induced colitis, and that Trpm8(-/-) CD11c+ DCs (dendritic cells) showed hyperinflammatory responses to toll-like receptor (TLR) stimulation. This was phenocopied in calcitonin gene-related peptide (CGRP) receptor-deficient mice, but not in substance P receptor-deficient mice, suggesting a functional link between TRPM8 and CGRP. The DSS phenotype of CGRP receptor-deficient mice could be adoptively transferred to wild-type (WT) mice, suggesting that CGRP suppresses the colitogenic activity of bone marrow-derived cells. TRPM8+ mucosal fibers expressed CGRP in human and mouse colon. Furthermore, neuronal CGRP contents were increased in colons from naive and DSS-treated Trpm8(-/-) mice, suggesting deficient CGRP release in the absence of TRPM8 triggering. Finally, treatment of Trpm8(-/-) mice with CGRP reversed their hyperinflammatory phenotype. These results suggest that TRPM8 signaling in mucosal sensory neurons is indispensable for the regulation of innate inflammatory responses via the neuropeptide CGRP. | 25269705
 |
Improving outcomes of acute kidney injury using mouse renal progenitor cells alone or in combination with erythropoietin or suramin.
Han, X; Zhao, L; Lu, G; Ge, J; Zhao, Y; Zu, S; Yuan, M; Liu, Y; Kong, F; Xiao, Z; Zhao, S
Stem cell research & therapy
4
74
2013
Kivonat megmutatása
So far, no effective therapy is available for acute kidney injury (AKI), a common and serious complication with high morbidity and mortality. Interest has recently been focused on the potential therapeutic effect of mouse adult renal progenitor cells (MRPC), erythropoietin (EPO) and suramin in the recovery of ischemia-induced AKI. The aim of the present study is to compare MRPC with MRPC/EPO or MRPC/suramin concomitantly in the treatment of a mouse model of ischemia/reperfusion (I/R) AKI.MRPC were isolated from adult C57BL/6-gfp mice. Male C57BL/6 mice (eight-weeks old, n = 72) were used for the I/R AKI model. Serum creatinine (Cr), blood urea nitrogen (BUN) and renal histology were detected in MRPC-, MRPC/EPO-, MRPC/suramin- and PBS-treated I/R AKI mice. E-cadherin, CD34 and GFP protein expression was assessed by immunohistochemical assay.MRPC exhibited characteristics consistent with renal stem cells. The features of MRPC were manifested by Pax-2, Oct-4, vimentin, α-smooth muscle actin positive, and E-cadherin negative, distinguished from mesenchymal stem cells (MSC) by expression of CD34 and Sca-1. The plasticity of MRPC was shown by the ability to differentiate into osteoblasts and lipocytes in vitro. Injection of MRPC, especially MRPC/EPO and MRPC/suramin in I/R AKI mice attenuated renal damage with a decrease of the necrotic injury, peak plasma Cr and BUN. Furthermore, seven days after the injury, MRPC/EPO or MRPC/suramin formed more CD34(+) and E-cadherin+ cells than MRPC alone.These results suggest that MRPC, in particular MRPC/EPO or MRPC/suramin, promote renal repair after injury and may be a promising therapeutic strategy. | 23777889
 |
The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion.
Comijn, J, et al.
Mol. Cell, 7: 1267-78 (2001)
2001
Kivonat megmutatása
Transcriptional downregulation of E-cadherin appears to be an important event in the progression of various epithelial tumors. SIP1 (ZEB-2) is a Smad-interacting, multi-zinc finger protein that shows specific DNA binding activity. Here, we report that expression of wild-type but not of mutated SIP1 downregulates mammalian E-cadherin transcription via binding to both conserved E2 boxes of the minimal E-cadherin promoter. SIP1 and Snail bind to partly overlapping promoter sequences and showed similar silencing effects. SIP1 can be induced by TGF-beta treatment and shows high expression in several E-cadherin-negative human carcinoma cell lines. Conditional expression of SIP1 in E-cadherin-positive MDCK cells abrogates E-cadherin-mediated intercellular adhesion and simultaneously induces invasion. SIP1 therefore appears to be a promoter of invasion in malignant epithelial tumors. | 11430829
 |
Inhibition of invasion of epithelial cells by Tiam1-Rac signaling.
Hordijk, P L, et al.
Science, 278: 1464-6 (1997)
1997
Kivonat megmutatása
Tiam1 encodes an exchange factor for the Rho-like guanosine triphosphatase Rac. Both Tiam1 and activated RacV12 promote invasiveness of T lymphoma cells. In epithelial Madin-Darby canine kidney (MDCK) cells, Tiam1 localized to adherens junctions. Ectopic expression of Tiam1 or RacV12 inhibited hepatocyte growth factor-induced scattering by increasing E-cadherin-mediated cell-cell adhesion accompanied by actin polymerization at cell-cell contacts. In Ras-transformed MDCK cells, expression of Tiam1 or RacV12 restored E-cadherin-mediated adhesion, resulting in phenotypic reversion and loss of invasiveness. These data suggest an invasion-suppressor role for Tiam1 and Rac in epithelial cells. | 9367959
 |
A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development.
Riethmacher, D, et al.
Proc. Natl. Acad. Sci. U.S.A., 92: 855-9 (1995)
1994
Kivonat megmutatása
The Ca(2+)-dependent cell adhesion molecule E-cadherin functions in the establishment and maintenance of epithelial cell morphology during embryogenesis and adulthood. Downregulation or complete shut-down of E-cadherin expression and mutation of the gene are observed during the progression of tumors of epithelial origin (carcinomas) and correlate with the metastatic potential. We have introduced a targeted mutation into the E-cadherin gene by homologous recombination in mouse embryonic stem cells. The mutation removes E-cadherin sequences essential for Ca2+ binding and for adhesive function. These embryonic stem cells were used to generate mice carrying the mutation. Heterozygous mutant animals appear normal and are fertile. However, the homozygous mutation is not compatible with life: E-cadherin -/- embryos show severe abnormalities before implantation. Particularly, the adhesive cells of the morula dissociate shortly after compaction has occurred, and their morphological polarization is then destroyed. Interestingly, the blastomers are still able to form desmosomes and tight junctions at sites of distorted cell-cell contact. Thus, maternal E-cadherin suffices for initial compaction of the morula but not for further preimplantation development to occur. | 7846066
 |
E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro.
Tang, A, et al.
J. Cell. Sci., 107 ( Pt 4): 983-92 (1994)
1993
Kivonat megmutatása
E- and P-cadherin are calcium (Ca2+)-dependent cell adhesion molecules important in the morphogenesis and maintenance of skin structure. By use of flow cytometry and specific antibodies, we now show that cultured human melanocytes express E- and P-cadherin on their surfaces, and that these molecules have the same characteristics as reported for other cell types. Specifically, melanocyte cadherins are sensitive to trypsin digestion in the absence of Ca2+ and are protected from trypsin degradation by Ca2+, and are functional at 37 degrees C but not at 4 degrees C. We further show that melanocytes contain mRNA transcripts encoding both E- and P-cadherin. Adhesion of cultured melanocytes to keratinocyte monolayers is abolished by pre-treatment of the melanocytes with trypsin/EDTA, which degrades E- and P-cadherins, is greatly reduced by anti-E-cadherin antibodies and is slightly reduced by antibodies to P-cadherin, alpha 2, alpha 3 and beta 1 integrins. In contrast to normal melanocytes, eight of nine melanoma cell lines lacked E-cadherin (or expressed markedly reduced levels) and five were negative for P-cadherin. Melanoma cells also failed to adhere to keratinocyte monolayers. These results demonstrate that normal human melanocytes express functional E- and P-cadherin and that E-cadherin is primarily responsible for adhesion of human melanocytes to keratinocytes in vitro. In addition, transformed melanocytes express markedly reduced levels of E- and P-cadherin, and exhibit decreased affinity for normal keratinocytes in vitro, suggesting that loss of cadherins may play a role in melanoma metastasis. | 8056851
 |