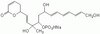
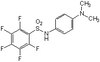
Suppression of eIF2α kinases alleviates Alzheimer's disease-related plasticity and memory deficits.
Ma, T; Trinh, MA; Wexler, AJ; Bourbon, C; Gatti, E; Pierre, P; Cavener, DR; Klann, E
Nature neuroscience
16
1299-305
2013
Kivonat megmutatása
Expression of long-lasting synaptic plasticity and long-term memory requires protein synthesis, which can be repressed by phosphorylation of eukaryotic initiation factor 2 α-subunit (eIF2α). Elevated phosphorylation of eIF2α has been observed in the brains of Alzheimer's disease patients and Alzheimer's disease model mice. Therefore, we tested whether suppressing eIF2α kinases could alleviate synaptic plasticity and memory deficits in Alzheimer's disease model mice. Genetic deletion of eIF2α kinase PERK prevented enhanced phosphorylation of eIF2α and deficits in protein synthesis, synaptic plasticity and spatial memory in mice that express familial Alzheimer's disease-related mutations in APP and PSEN1. Similarly, deletion of another eIF2α kinase, GCN2, prevented impairments of synaptic plasticity and defects in spatial memory exhibited by the Alzheimer's disease model mice. Our findings implicate aberrant eIF2α phosphorylation as a previously unidentified molecular mechanism underlying Alzheimer's disease-related synaptic pathophysioloy and memory dysfunction and suggest that PERK and GCN2 are potential therapeutic targets for treatment of individuals with Alzheimer's disease. | 23933749
 |
Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium.
Hoch, B, et al.
Circ. Res., 84: 713-21 (1999)
1998
Kivonat megmutatása
Despite its importance for the regulation of heart function, little is known about the isoform expression of the multifunctional Ca2+/calmodulin-dependent protein kinase (CaMKII) in human myocardium. In this study, we investigated the spectrum of CaMKII isoforms delta2, delta3, delta4, delta8, and delta9 in human striated muscle tissue. Isoform delta3 is characteristically expressed in cardiac muscle. In skeletal muscle, specific expression of a new isoform termed delta11 is demonstrated. Complete sequencing of human delta2 cDNA, representing all common features of the investigated CaMKII subclass, revealed its high homology to the corresponding rat cDNA. Comparative semiquantitative reverse transcription-polymerase chain reaction analyses from left ventricular tissues of normal hearts and from patients suffering from dilated cardiomyopathy showed a significant increase in transcript levels of isoform delta3 relative to the expression of glyceraldehyde-3-phosphate dehydrogenase in diseased hearts (101. 6+/-11.0% versus 64.9+/-9.9% in the nonfailing group; P<0.05, n=6). Transcript levels of the other investigated cardiac CaMKII isoforms remained unchanged. At the protein level, by using a subclass-specific antibody, we observed a similar increase of a delta-CaMKII-specific signal (7.2+/-1.0 versus 3.8+/-0.7 optical density units in the nonfailing group; P<0.05, n=4 through 6). The diseased state of the failing hearts was confirmed by a significant increase in transcript levels for atrial natriuretic peptide (292. 9+/-76.4% versus 40.1+/-3.2% in the nonfailing group; P<0.05, n=3 through 6). Our data characterize for the first time the delta-CaMKII isoform expression pattern in human hearts and demonstrate changes in this expression pattern in heart failure. | 10189359
 |
Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation.
Koh, Y H, et al.
Cell, 98: 353-63 (1999)
1998
Kivonat megmutatása
Discs large (DLG) mediates the clustering of synaptic molecules. Here we demonstrate that synaptic localization of DLG itself is regulated by CaMKII. We show that DLG and CaMKII colocalize at synapses and exist in the same protein complex. Constitutively activated CaMKII phenocopied structural abnormalities of dlg mutant synapses and dramatically increased extrajunctional DLG. Decreased CaMKII activity caused opposite alterations. In vitro, CaMKII phosphorylated a DLG fragment with a stoichiometry close to one. Moreover, expression of site-directed dlg mutants that blocked or mimicked phosphorylation had effects similar to those observed upon inhibiting or constitutively activating CaMKII. We propose that CaMKII-dependent DLG phosphorylation regulates the association of DLG with the synaptic complex during development and plasticity, thus providing a link between synaptic activity and structure. | 10458610
 |
AlphaCaMKII binding to the C-terminal tail of NMDA receptor subunit NR2A and its modulation by autophosphorylation.
Gardoni, F, et al.
FEBS Lett., 456: 394-8 (1999)
1998
| 10462051
 |
Downregulation of delta CaM kinase II in human tumor cells.
Tombes, R M, et al.
Biochim. Biophys. Acta, 1452: 1-11 (1999)
1998
Kivonat megmutatása
Over two dozen alternative splice variants of CaMK-II, the type II Ca(2+)/CaM-dependent protein kinase, are encoded from four genes (alpha, beta, gamma and delta) in mammalian cells. Isozymes of alpha and beta CaMK-II are well characterized in brain; however, an understanding of the relative endogenous levels of CaMK-II isozymes in a wide variety of non-neuronal cells has not yet been described. In this study, we have demonstrated that CaMK-II consists primarily of the 54 kDa delta CaMK-II (delta(2) or delta(C)) isozyme in rodent fibroblasts. beta and gamma CaMK-II isozymes are minor and alpha CaMK-II was not expressed. The primary delta CaMK-II in human fibroblasts and the MCF10A mammary epithelial cell line was the 52 kDa delta(4) CaMK-II, an isozyme identical to delta(2) except for a missing 21-amino-acid C-terminal tail. delta CaMK-II levels were diminished in both human and rodent fibroblasts after SV40 transformation and in the mammary adenocarcinoma MCF7 cell line when compared to MCF10A cells. In fact, most tumor cells exhibited CaMK-II specific activities which were two- to tenfold lower than in untransformed fibroblasts. We conducted complementary CaMK-II studies on the NGF-induced differentiation of rat PC-12 cells. Although no new synthesis of CaMK-II occurs, neurite outgrowth in these cells is accompanied by a preferential activation of delta CaMK-II. Endogenous delta CaMK-II has a perinuclear distribution in fibroblasts and extends along neurites in PC-12 cells. These findings point to a role for delta CaMK-II isozymes in cellular differentiation. | 10525155
 |
Inhibition of calcium/calmodulin-dependent protein kinase II in rat hippocampus attenuates morphine tolerance and dependence.
Fan, G H, et al.
Mol. Pharmacol., 56: 39-45 (1999)
1998
Kivonat megmutatása
Learning and memory have been suggested to be important in the development of opiate addiction. Based on the recent findings that calcium/calmodulin-dependent protein kinase II (CaMKII) is essential in learning and memory processes, and morphine treatment increases CaMKII activity in hippocampus, the present study was undertaken to examine whether inhibition of hippocampal CaMKII prevents morphine tolerance and dependence. Here, we report that inhibition of CaMKII by intrahippocampal dentate gyrus administration of the specific inhibitors KN-62 and KN-93 to rats significantly attenuated the tolerance to the analgesic effect of morphine and the abstinence syndrome precipitated by opiate antagonist naloxone. In contrast, both KN-04 and KN-92, the inactive structural analogs of KN-62 and KN-93, failed to attenuate morphine tolerance and dependence, indicating that the observed effects of KN-62 and KN-93 are mediated through inhibition of CaMKII. Furthermore, administration of CaMKII antisense oligonucleotide into rat hippocampal dentate gyrus, which decreased the expression of CaMKII specifically, also attenuated morphine tolerance and dependence, while the corresponding sense oligonucleotide of CaMKII did not exhibit such inhibitory effect. Moreover, the KN-62 treatment abolished the rewarding properties of morphine as measured by the conditioned place preference. These results suggest that hippocampal CaMKII is critically involved in the development of morphine tolerance and dependence, and inhibition of this kinase may have some therapeutic benefit in the treatment of opiate tolerance and dependence. | 10385682
 |
CaMKII regulates the density of central glutamatergic synapses in vivo.
Rongo, C and Kaplan, J M
Nature, 402: 195-9 (1999)
1998
| 10647013
 |
Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain.
Erondu, N E and Kennedy, M B
J. Neurosci., 5: 3270-7 (1985)
1985
Kivonat megmutatása
The distribution of type II Ca2+/calmodulin-dependent protein kinase has been mapped in rat brain by immunochemical and immunohistochemical methods using an antibody against its alpha-subunit. The concentration of the kinase, measured by radioimmunoassay, varies markedly in different brain regions. It is most highly concentrated in the telencephalon where it comprises approximately 2% of the total hippocampal protein, 1.3% of cortical protein, and 0.7% of striatal protein. It is less concentrated in lower brain structures, ranging from about 0.3% of hypothalamic protein to 0.1% of protein in the pons/medulla. The gradient of staining intensity observed in brain sections by immunohistochemistry corroborates this distribution. Neurons and neuropil of the hippocampus are densely stained, whereas little staining is observed in lower brain regions such as the superior colliculus. Within the diencephalon and midbrain, dense staining is observed only in thalamic nuclei and the substantia nigra. The skewed distribution of alpha-subunit appears to be due in part to the occurrence in the cerebellum and pons/medulla of forms of the kinase with a high ratio of beta- to alpha-subunits. However, most of the variation is due to the extremely high concentration of the kinase in particular neurons, especially those of the hippocampus, cortex and striatum. The unusually high expression of the kinase in these neurons is likely to confer upon them specialized responses to calcium ion that are different from those of neurons in lower brain regions. | 4078628
 |