401003 Sigma-Aldrich(±)-Ibuprofen - CAS 15687-27-1 - Calbiochem
A nonsteroidal anti-inflammatory drug (NSAID) that acts as a reversible and competitive inhibitor of cyclooxygenase 1 (COX-1) (IC₅₀ = 4.85 µM).
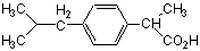
More>> A nonsteroidal anti-inflammatory drug (NSAID) that acts as a reversible and competitive inhibitor of cyclooxygenase 1 (COX-1) (IC₅₀ = 4.85 µM). Less<<Synonyms: [(±)-2-(4-Isobutylphenyl)-propionic Acid
Recommended Products
Áttekintés
| Replacement Information |
|---|
Kulcsspecifikációk táblázata
| CAS # | Empirical Formula |
|---|---|
| 15687-27-1 | C₁₃H₁₈O₂ |
Products
| Katalógusszám | Csomagolás | Menny./csomag | |
|---|---|---|---|
| 401003-1GM | Muanyagampulla | 1 gm |
| Product Information | |
|---|---|
| CAS number | 15687-27-1 |
| ATP Competitive | Y |
| Form | White solid |
| Hill Formula | C₁₃H₁₈O₂ |
| Chemical formula | C₁₃H₁₈O₂ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | COX-1 |
| Primary Target IC<sub>50</sub> | 4.85 µM against COX-1 |
| Purity | ≥98% by titration |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | MU6640000 |
| Safety Information | |
|---|---|
| R Phrase | R: 22 Harmful if swallowed. |
| S Phrase | S: 36 Wear suitable protective clothing. |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalógusszám | GTIN |
| 401003-1GM | 04055977189377 |
Documentation
(±)-Ibuprofen - CAS 15687-27-1 - Calbiochem MSDS
| Title |
|---|
(±)-Ibuprofen - CAS 15687-27-1 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 401003 |
References
| Hivatkozások áttekintése |
|---|
| Asanuma, M., et al. 2001. J. Neurochem. 76, 1895. Blasko, I., et al. 2001. Neurobiol. Dis. 8, 1094. Ouellet, M., et al. 2001. Proc. Natl. Acad. Sci. USA 98, 14583. Casper, D., et al. 2000. Neurosci. Lett. 289, 201. Lambat, Z., et al. 2000. Metab. Brain Dis. 15, 249. Lim, G.P., et al. 2000. J. Neurosci. 20, 5709. Ogawa, O., et al. 2000. Eur. J. Pharmacol. 408, 137. Wyss-Coray, T., and Mucke, L. 2000. Nat. Med. 6, 973. Lehmann, J.M., et al. 1997. J. Biol. Chem. 272, 3406. Boneburg, E.M., et al. 1996. J. Clin. Pharmacol. 36, 16S. Mitchell, J.A., et al. 1994. Proc. Natl. Acad. Sci. USA 90, 11693. |