574625 Sigma-AldrichSuramin, Sodium Salt - CAS 129-46-4 - Calbiochem
A reversible and competitive inhibitor of protein tyrosine phosphatases.
More>> A reversible and competitive inhibitor of protein tyrosine phosphatases. Less<<MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents.
同义词: P2Y Antagonist I, Purinergic Receptor P2Y Antagonist I, PTP Inhibitor VI
Recommended Products
概述
| Replacement Information |
|---|
重要规格表
| CAS # | Empirical Formula |
|---|---|
| 129-46-4 | C₅₁H₃₄N₆O₂₃S₆ . 6Na |
价格及供货情况
| 产品目录编号 | 库存情况 | 包装 | 数量 / 包装 | 价格 | 数量 | |
|---|---|---|---|---|---|---|
| 574625-50MGCN |
|
塑胶安瓿;塑胶针药瓶 | 50 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 129-46-4 |
| ATP Competitive | N |
| Form | White to beige to peach crystalline solid |
| Hill Formula | C₅₁H₃₄N₆O₂₃S₆ . 6Na |
| Chemical formula | C₅₁H₃₄N₆O₂₃S₆ . 6Na |
| Hygroscopic | Hygroscopic |
| Reversible | Y |
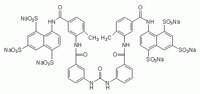
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | protein tyrosine phosphatases |
| Primary Target IC<sub>50</sub> | 15 µM against phospholipase D |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | QM7000000 |
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| 产品目录编号 | GTIN |
| 574625-50MGCN | 04055977189636 |
Documentation
Suramin, Sodium Salt - CAS 129-46-4 - Calbiochem MSDS
| 职位 |
|---|
Suramin, Sodium Salt - CAS 129-46-4 - Calbiochem 分析证书
| 标题 | 批号 |
|---|---|
| 574625 |
参考
| 参考信息概述 |
|---|
| Meyers, M.O., et al. 2000. J. Surg. Res. 91, 130. Hohenegger, M., et al. 1998. Proc. Natl. Acad. Sci. USA 95, 346. Waldhoer, M., et al. 1998. Mol. Pharmacol. 53, 808. Zhang, Y.L., et al. 1998. J. Biol. Chem. 273, 12281. Hohenegger, M., et al. 1996. Mol. Pharmacol. 50, 1443. Emmick, J.J., et al. 1994. J. Pharmacol. Exp. Ther. 269, 717. Denhertog, A., et al. 1992. J. Physiol. 454, 591. Nakajima, M., et al. 1991. J. Biol. Chem. 266, 9661. Wilks, J.W., et al. 1991. Int. J. Radiat. Biol. 60, 73. Huang, R.C., et al. 1990. Mol. Pharmacol. 37, 304. Kopp, R. and Pfeiffer, A. 1990. Cancer Res. 50, 6490. |
数据表
| 标题 |
|---|
| SIRTainty™ Class III HDAC Assay |