03-0193-00 MilliporeSMC™ Human SARS-CoV-2 RBD IgG Kit
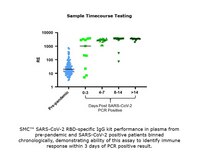
The sensitivity of the Single Molecule Counting (SMC™) technology gives the SMC™ SARS-CoV-2 RBD IgG Kit unparalleled performance in the qualitative determination of SARS-CoV-2 RBD IgG in Human EDTA Plasma and Serum.
More>> The sensitivity of the Single Molecule Counting (SMC™) technology gives the SMC™ SARS-CoV-2 RBD IgG Kit unparalleled performance in the qualitative determination of SARS-CoV-2 RBD IgG in Human EDTA Plasma and Serum. Less<<SMC™ Human SARS-CoV-2 RBD IgG Kit MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents.
Recommended Products
概述
| Replacement Information |
|---|
重要规格表
| Key Applications |
|---|
| Single Molecule Counting (SMC®) |
| References |
|---|
| Biological Information |
|---|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Storage and Shipping Information |
|---|
| Packaging Information | |
|---|---|
| Material Size | 96 well assay |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| 产品目录编号 | GTIN |
| 03-0193-00 | 04061841868415 |
Documentation
Protocols
| Title |
|---|
| Protocol- 03-0193-00 |
| SMC™ Human SARS-CoV-2 RBD IgG Kit |
SMC™ Human SARS-CoV-2 RBD IgG Kit MSDS
| 职位 |
|---|