533660 Sigma-AldrichLactate Dehydrogenase Inhibitor II, GSK2837808A
A cell-permeable, NADH-competitive LDHA selective inhibitor (IC₅₀ = 2.6 & 43 nM for LDHA & LDHB). Blocks cytosolic glycolysis, activates PKM2 and induces apoptosis.
More>> A cell-permeable, NADH-competitive LDHA selective inhibitor (IC₅₀ = 2.6 & 43 nM for LDHA & LDHB). Blocks cytosolic glycolysis, activates PKM2 and induces apoptosis. Less<<MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents.
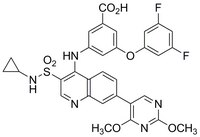
同义词: LDH Inhibitor II, Compound 1, 3-((3-(N-Cyclopropylsulfamoyl)-7-(2,4-dimethoxypyrimidin-5-yl)quinolin-4-yl)amino)-5-(3,5-difluorophenoxy)benzoic acid
Recommended Products
概述
| Replacement Information |
|---|
重要规格表
| Empirical Formula |
|---|
| C₃₁H₂₅F₂N₅O₇S |
价格及供货情况
| 产品目录编号 | 库存情况 | 包装 | 数量 / 包装 | 价格 | 数量 | |
|---|---|---|---|---|---|---|
| 5.33660.0001 |
|
玻璃瓶 | 5 mg |
|
— |
| References | |
|---|---|
| References | Xie, H. 2014. Cell Metab. 19, 795. Billiard, J. 2013. Cancer Metab. 1, 19. |
| Product Information | |
|---|---|
| Form | Yellow solid |
| Hill Formula | C₃₁H₂₅F₂N₅O₇S |
| Chemical formula | C₃₁H₂₅F₂N₅O₇S |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | lactate dehydrogenase A |
| Primary Target IC<sub>50</sub> | 2.6 nM for human LDH-A |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| 产品目录编号 | GTIN |
| 5.33660.0001 | 04055977286601 |
Documentation
Lactate Dehydrogenase Inhibitor II, GSK2837808A MSDS
| 职位 |
|---|
参考
| 参考信息概述 |
|---|
| Xie, H. 2014. Cell Metab. 19, 795. Billiard, J. 2013. Cancer Metab. 1, 19. |