341006 Sigma-AldrichEDAC, Hydrochloride
EDAC HCl is a water-soluble derivative of carbodiimide useful for conjugating haptens to proteins and polypeptides. Used to modify NMDA receptors and as a condensing agent in peptide synthesis.
More>> EDAC HCl is a water-soluble derivative of carbodiimide useful for conjugating haptens to proteins and polypeptides. Used to modify NMDA receptors and as a condensing agent in peptide synthesis. Less<<EDAC, Hydrochloride MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents.
同义词: EDCI, 1-Ethyl-3-(3ʹ-dimethylaminopropyl)carbodiimide, HCl
Recommended Products
概述
| Replacement Information |
|---|
重要规格表
| CAS # | Purity |
|---|---|
| 25952-53-8 | ≥98% by Titration |
价格及供货情况
| 产品目录编号 | 库存情况 | 包装 | 数量 / 包装 | 价格 | 数量 | |
|---|---|---|---|---|---|---|
| 341006-25GMCN |
|
玻璃瓶 | 25 gm |
|
— | |
| 341006-5GMCN |
|
塑胶安瓿;塑胶针药瓶 | 5 gm |
|
— |
| Product Information | |
|---|---|
| CAS number | 25952-53-8 |
| Form | White solid |
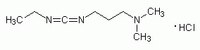
| Hill Formula | C₈H₁₇N₃ · HCl |
| Chemical formula | C₈H₁₇N₃ · HCl |
| Hygroscopic | Hygroscopic |
| Structure formula Image | |
| Quality Level | MQ200 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by Titration |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | FF2200000 |
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| 产品目录编号 | GTIN |
| 341006-25GMCN | 07790788054182 |
| 341006-5GMCN | 04055977215403 |
Documentation
EDAC, Hydrochloride MSDS
| 职位 |
|---|
EDAC, Hydrochloride 分析证书
| 标题 | 批号 |
|---|---|
| 341006 |
参考
| 参考信息概述 |
|---|
| Chazot, P.L., et al. 1993. Biochem. Pharmacol. 45, 605. Richardson, A., et al. 1992. Biochem. Pharmacol. 43, 1415. Taniuchi, M., et al. 1986. Proc. Natl. Acad. Sci. USA 83, 1950. Chase, J.W., et al. 1983. Proc. Natl. Acad. Sci. USA 80, 5480. Williams, A., et al. 1981. J. Am. Chem. Soc. 103, 7090. Yamada, H., et al. 1981. Biochemistry 20, 4836. Thomas, J.O., et al. 1978. J. Mol. Biol. 123, 149. Ozawa, H. 1970. Biochemistry 9, 2158. Kopple, K.D., et al. 1962. J. Am. Chem. Soc. 84, 4457. |
小册子
| 标题 |
|---|
| Excitotoxic Glutamate Analogs Technical Bulletin |