196440 Sigma-AldrichBatimastat - CAS 130370-60-4 - Calbiochem
Batimastat, CAS 130370-60-4, is a potent inhibitor of a several metalloproteinases, including MMP-1, 2, 3, 7, 9, ΔMT1, ADAM8 & ADAM17/TACE (IC50 = 3, 4, 20, 6, 4, 2.08, 51.3 & 19 nM, respectiv
More>> Batimastat, CAS 130370-60-4, is a potent inhibitor of a several metalloproteinases, including MMP-1, 2, 3, 7, 9, ΔMT1, ADAM8 & ADAM17/TACE (IC50 = 3, 4, 20, 6, 4, 2.08, 51.3 & 19 nM, respectively). Less<<Batimastat - CAS 130370-60-4 - Calbiochem MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents.
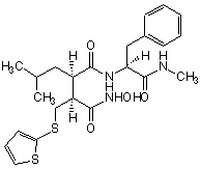
同义词: (4-N-Hydroxyamino)-2R-isobutyl-3S-(thienylthiomethyl)succinyl)-L-phenylalanine-N-methylamide, BB-94
Recommended Products
概述
重要规格表
| CAS # | Empirical Formula |
|---|---|
| 130370-60-4 | C₂₃H₃₁N₃O₄S₂ |
价格及供货情况
| 产品目录编号 | 库存情况 | 包装 | 数量 / 包装 | 价格 | 数量 | |
|---|---|---|---|---|---|---|
| 196440-5MGCN |
|
玻璃瓶 | 5 mg |
|
— |
| Description | |
|---|---|
| Overview | A Marimastat (Cat. No. 444289) type of peptidyl hydroxamate-based inhibitor that potently inhibits a broad-spectrum of metalloproteinases, including MMP-1, MMP-2, MMP-3/stromelysin, MMP-7/matrilysin, MMP-9, ΔMT1 (MMP-14 without TM domain), ADAM8, and ADAM17/TACE (IC50 = 3, 4, 20, 6, 4, 2.08, 51.3, and 19 nM, respectively), by targeting both the substrate binding site and the active-site Zn2+, while being much less potent toward ACE (Angiotensin Converting Enzyme) or α-secretase (IC50 = 1.6 and 3.3 µM, respectively). Batimastat is widely used in studying the involvement of MMPs in cancinogenesis and non-cancer pathological processes both in cultures in vitro and in animals in vivo. Also available as a 25 mM solution in DMSO (Cat. No. 508408). |
| Catalogue Number | 196440 |
| Brand Family | Calbiochem® |
| Synonyms | (4-N-Hydroxyamino)-2R-isobutyl-3S-(thienylthiomethyl)succinyl)-L-phenylalanine-N-methylamide, BB-94 |
| Product Information | |
|---|---|
| CAS number | 130370-60-4 |
| Form | Off-white solid |
| Hill Formula | C₂₃H₃₁N₃O₄S₂ |
| Chemical formula | C₂₃H₃₁N₃O₄S₂ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Global Trade Item Number | |
|---|---|
| 产品目录编号 | GTIN |
| 196440-5MGCN | 04055977206500 |