196876 Sigma-AldrichBAY 41-2272 - CAS 256376-24-6 - Calbiochem
A cell-permeable pyrazolopyridinylpyrimidine compound that acts as a selective and potent stimulator of soluble guanylate cyclase (effective dose ~ 0.1 nM to 100 µM using recombinant soluble guanylate cyclase).
More>> A cell-permeable pyrazolopyridinylpyrimidine compound that acts as a selective and potent stimulator of soluble guanylate cyclase (effective dose ~ 0.1 nM to 100 µM using recombinant soluble guanylate cyclase). Less<<BAY 41-2272 - CAS 256376-24-6 - Calbiochem MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents.
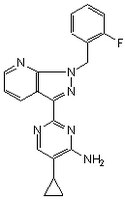
同义词: 5-Cyclopropyl-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-4-ylamine
Recommended Products
概述
| Replacement Information |
|---|
重要规格表
| CAS # | Empirical Formula |
|---|---|
| 256376-24-6 | C₂₀H₁₇FN₆ |
价格及供货情况
| 产品目录编号 | 库存情况 | 包装 | 数量 / 包装 | 价格 | 数量 | |
|---|---|---|---|---|---|---|
| 196876-5MGCN |
|
塑胶安瓿;塑胶针药瓶 | 5 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 256376-24-6 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₂₀H₁₇FN₆ |
| Chemical formula | C₂₀H₁₇FN₆ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| 产品目录编号 | GTIN |
| 196876-5MGCN | 04055977206593 |
Documentation
BAY 41-2272 - CAS 256376-24-6 - Calbiochem MSDS
| 职位 |
|---|
BAY 41-2272 - CAS 256376-24-6 - Calbiochem 分析证书
| 标题 | 批号 |
|---|---|
| 196876 |
参考
| 参考信息概述 |
|---|
| Boerrigter, G., et al. 2003. Circulation 107, 686. Kalsi, J.S., et al. 2003. J Urol. 169, 761. Koglin, M., et al. 2002. Biochem. Biophys. Res. Commun. 292, 1057. Becker, E.M., et al. 2001. BMC Pharmacol. 1, 13. Stasch, J.P., et al. 2001. Nature 410, 212. |