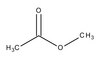
Sustained therapeutic hypercapnia attenuates pulmonary arterial Rho-kinase activity and ameliorates chronic hypoxic pulmonary hypertension in juvenile rats.
Peng, G; Ivanovska, J; Kantores, C; Van Vliet, T; Engelberts, D; Kavanagh, BP; Enomoto, M; Belik, J; Jain, A; McNamara, PJ; Jankov, RP
American journal of physiology. Heart and circulatory physiology
302
H2599-611
2012
显示摘要
Sustained therapeutic hypercapnia prevents pulmonary hypertension in experimental animals, but its rescue effects on established disease have not been studied. Therapies that inhibit Rho-kinase (ROCK) and/or augment nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) signaling can reverse or prevent progression of chronic pulmonary hypertension. Our objective in the present study was to determine whether sustained rescue treatment with inhaled CO(2) (therapeutic hypercapnia) would improve structural and functional changes of chronic hypoxic pulmonary hypertension. Spontaneously breathing pups were exposed to normoxia (21% O(2)) or hypoxia (13% O(2)) from postnatal days 1-21 with or without 7% CO(2) (Pa(CO(2)) elevated by ∼25 mmHg) or 10% CO(2) (Pa(CO(2)) elevated by ∼40 mmHg) from days 14 to 21. Compared with hypoxia alone, animals exposed to hypoxia and 10% CO(2) had significantly (P less than 0.05) decreased pulmonary vascular resistance, right-ventricular systolic pressure, right-ventricular hypertrophy, and medial wall thickness of pulmonary resistance arteries as well as decreased lung phosphodiesterase (PDE) V, RhoA, and ROCK activity. Rescue treatment with 10% CO(2), or treatment with a ROCK inhibitor (15 mg/kg ip Y-27632 twice daily from days 14 to 21), also increased pulmonary arterial endothelial nitric oxide synthase and lung NO content. In contrast, cGMP content and cGMP-dependent protein kinase (PKG) activity were increased by exposure to 10% CO(2), but not by ROCK inhibition with Y-27632. In vitro exposure of pulmonary artery smooth muscle cells to hypercapnia suppressed serum-induced ROCK activity, which was prevented by inhibition of PKG with Rp-8-Br-PET-cGMPS. We conclude that sustained hypercapnia dose-dependently inhibited ROCK activity, augmented NO-cGMP-PKG signaling, and led to partial improvements in the hemodynamic and structural abnormalities of chronic hypoxic PHT in juvenile rats. Increased PKG content and activity appears to play a major upstream role in CO(2)-induced suppression of ROCK activity in pulmonary arterial smooth muscle. | 22505643
 |
The vitamin D receptor agonist elocalcitol inhibits IL-8-dependent benign prostatic hyperplasia stromal cell proliferation and inflammatory response by targeting the RhoA/Rho kinase and NF-kB pathways.
Giuseppe Penna, Benedetta Fibbi, Susana Amuchastegui, Elisa Corsiero, Gilles Laverny, Enrico Silvestrini, Aravinda Chavalmane, Annamaria Morelli, Erica Sarchielli, Gabriella Barbara Vannelli, Mauro Gacci, Enrico Colli, Mario Maggi, Luciano Adorini, Giuseppe Penna, Benedetta Fibbi, Susana Amuchastegui, Elisa Corsiero, Gilles Laverny, Enrico Silvestrini, Aravinda Chavalmane, Annamaria Morelli, Erica Sarchielli, Gabriella Barbara Vannelli, Mauro Gacci, Enrico Colli, Mario Maggi, Luciano Adorini, Giuseppe Penna, Benedetta Fibbi, Susana Amuchastegui, Elisa Corsiero, Gilles Laverny, Enrico Silvestrini, Aravinda Chavalmane, Annamaria Morelli, Erica Sarchielli, Gabriella Barbara Vannelli, Mauro Gacci, Enrico Colli, Mario Maggi, Luciano Adorini
The Prostate
69
480-93
2009
显示摘要
BACKGROUND: Benign prostatic hyperplasia (BPH) is characterized by an important inflammatory component. Stimulation of human prostate stromal cells from BPH tissues with proinflammatory cytokines leads to secretion of IL-8, a chemokine involved in BPH pathogenesis. The vitamin D receptor (VDR) agonist elocalcitol can arrest prostate growth in BPH patients, but its mechanism of action in this pathology is still incompletely understood. METHODS: IL-8 levels were measured by real-time RT-PCR and ELISA. NF-kappaB translocation and COX-2 expression were evaluated by confocal microscopy. RhoA and Rho-kinase (ROCK) gene expression and functional activity were studied by real-time RT-PCR, immuno-kinase assays, Western blot analysis, confocal microscopy, and cell invasion. RESULTS: Stimulation of BPH cells with IL-8 activates the calcium-sensitizing RhoA/ROCK pathway, as demonstrated by the increased membrane translocation of RhoA and by phosphorylation of the ROCK substrate myosin phosphatase target subunit 1 (MYPT-1). In agreement with these data, C3 exoenzyme, a selective RhoA inhibitor, inhibits IL-8-induced invasion of BPH cells. The VDR agonist elocalcitol significantly inhibits IL-8 production by BPH cells stimulated with inflammatory cytokines, and IL-8-induced proliferation of BPH cells. In addition, elocalcitol inhibits IL-8-induced membrane translocation of RhoA and MYPT-1 phosphorylation in BPH cells, and inhibits dose-dependently their IL-8-dependent invasion. The inhibition induced by elocalcitol of IL-8 production by BPH cells is accompanied by decreased COX-2 expression and PGE(2) production and by arrest of NF-kappaB p65 nuclear translocation, associated with inhibition of the RhoA/ROCK pathway. CONCLUSIONS: These data provide a mechanistic explanation for the anti-proliferative and anti-inflammatory properties of elocalcitol in BPH cells. Prostate 69:480-493, 2009. (c) 2008 Wiley-Liss, Inc. | 19107880
 |
Myosin phosphatase: structure, regulation and function.
Ito, Masaaki, et al.
Mol. Cell. Biochem., 259: 197-209 (2004)
2004
显示摘要
Phosphorylation of myosin II plays an important role in many cell functions, including smooth muscle contraction. The level of myosin II phosphorylation is determined by activities of myosin light chain kinase and myosin phosphatase (MP). MP is composed of 3 subunits: a catalytic subunit of type 1 phosphatase, PPlc; a targeting subunit, termed myosin phosphatase target subunit, MYPT; and a smaller subunit, M20, of unknown function. Most of the properties of MP are due to MYPT and include binding of PP1c and substrate. Other interactions are discussed. A recent discovery is the existence of an MYPT family and members include, MYPT1, MYPT2, MBS85, MYPT3 and TIMAP. Characteristics of each are outlined. An important discovery was that the activity of MP could be regulated and both activation and inhibition were reported. Activation occurs in response to elevated cyclic nucleotide levels and various mechanisms are presented. Inhibition of MP is a major component of Ca2+-sensitization in smooth muscle and various molecular mechanisms are discussed. Two mechanisms are cited frequently: (1) Phosphorylation of an inhibitory site on MYPT1, Thr696 (human isoform) and resulting inhibition of PP1c activity. Several kinases can phosphorylate Thr696, including Rho-kinase that serves an important role in smooth muscle function; and (2) Inhibition of MP by the protein kinase C-potentiated inhibitor protein of 17 kDa (CPI-17). Examples where these mechanisms are implicated in smooth muscle function are presented. The critical role of RhoA/Rho-kinase signaling in various systems is discussed, in particular those vascular smooth muscle disorders involving hypercontractility. | 15124925
 |
Hypertension and RhoA/Rho-kinase signaling in the vasculature: highlights from the recent literature.
Lee, Dexter L, et al.
Hypertension, 44: 796-9 (2004)
2004
显示摘要
Under normal conditions, contractile activity in vascular smooth muscle is initiated by either receptor activation (norepinephrine, angiotensin II, etc.) or by a stretch-activated mechanism. After this activation, several signaling pathways can initiate a Ca2+-calmodulin interaction to stimulate phosphorylation of the light chain of myosin. Ca2+ sensitization of the contractile proteins is signaled by the RhoA/Rho-kinase pathway to inhibit the dephosphorylation of the light chain by myosin phosphatase thereby maintaining force generation. In opposition to force generation, NO is released from endothelial cells and causes vasodilation through inhibition of the RhoA/Rho-kinase signaling pathway. This brief review will highlight recent studies demonstrating a role for the RhoA/Rho-kinase signaling pathway in the increased vasoconstriction characteristic of hypertension. | 15520302
 |
RhoA-mediated Ca2+ sensitization in erectile function.
Wang, Hua, et al.
J. Biol. Chem., 277: 30614-21 (2002)
2002
显示摘要
A Rho-kinase inhibitor increases corpus cavernosum (CC) pressure in an in vivo rat model (Chitaley, K., Wingard, C. J., Webb, R. C., Branam, H., Stopper, V. S., Lewis, R. W., and Mills, T. M. (2001) Nat. Med. 7, 119-122) suggesting that Rho-mediated Ca(2+) sensitization of CC smooth muscle maintains the flaccid (contracted) state. We directly demonstrate Ca(2+) sensitization of permeabilized rabbit and human CC and identify a highly expressed molecular component of this pathway. Ca(2+) sensitization of force induced by endothelin or GTPgammaS was significantly greater in CC than in rabbit ileum smooth muscle and was accompanied by a 17-fold higher RhoA content. Pull-down assays with the RhoA binding domain of mDia showed the high RhoA content of CC to be available for activation by GTPgammaS. Ca(2+) sensitization induced by endothelin, phenylephrine, or GTPgammaS was completely relaxed by the Rho kinase inhibitor Y-27632. Human and rabbit CC both express the phosphatase inhibitor CPI-17, the myosin phosphatase regulatory (MYPT-1) and catalytic (PP1delta) subunits, and two isoforms of Rho kinase. We suggest that high expression of RhoA contributes, through RhoA-mediated Ca(2+) sensitization, to the flaccid state of CC that can be reversed by a water-soluble, orally active Rho kinase inhibitor suitable for therapy of erectile dysfunction. | 12060659
 |