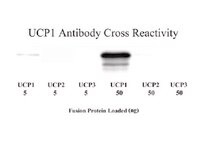
3,5-Diiodo-L-thyronine activates brown adipose tissue thermogenesis in hypothyroid rats.
Lombardi, A; Senese, R; De Matteis, R; Busiello, RA; Cioffi, F; Goglia, F; Lanni, A
PloS one
10
e0116498
2015
显示摘要
3,5-Diiodo-l-thyronine (T2), a thyroid hormone derivative, is capable of increasing energy expenditure, as well as preventing high fat diet-induced overweight and related metabolic dysfunction. Most studies to date on T2 have been carried out on liver and skeletal muscle. Considering the role of brown adipose tissue (BAT) in energy and metabolic homeostasis, we explored whether T2 could activate BAT thermogenesis. Using euthyroid, hypothyroid, and T2-treated hypothyroid rats (all maintained at thermoneutrality) in morphological and functional studies, we found that hypothyroidism suppresses the maximal oxidative capacity of BAT and thermogenesis, as revealed by reduced mitochondrial content and respiration, enlarged cells and lipid droplets, and increased number of unilocular cells within the tissue. In vivo administration of T2 to hypothyroid rats activated BAT thermogenesis and increased the sympathetic innervation and vascularization of tissue. Likewise, T2 increased BAT oxidative capacity in vitro when added to BAT homogenates from hypothyroid rats. In vivo administration of T2 to hypothyroid rats enhanced mitochondrial respiration. Moreover, UCP1 seems to be a molecular determinant underlying the effect of T2 on mitochondrial thermogenesis. In fact, inhibition of mitochondrial respiration by GDP and its reactivation by fatty acids were greater in mitochondria from T2-treated hypothyroid rats than untreated hypothyroid rats. In vivo administration of T2 led to an increase in PGC-1α protein levels in nuclei (transient) and mitochondria (longer lasting), suggesting a coordinate effect of T2 in these organelles that ultimately promotes net activation of mitochondrial biogenesis and BAT thermogenesis. The effect of T2 on PGC-1α is similar to that elicited by triiodothyronine. As a whole, the data reported here indicate T2 is a thyroid hormone derivative able to activate BAT thermogenesis. | 25658324
 |
Liver kinase b1 is required for white adipose tissue growth and differentiation.
Zhang, W; Wang, Q; Song, P; Zou, MH
Diabetes
62
2347-58
2013
显示摘要
White adipose tissue (WAT) is not only a lipogenic and fat storage tissue but also an important endocrine organ that regulates energy homeostasis, lipid metabolism, appetite, fertility, and immune and stress responses. Liver kinase B1 (LKB1), a tumor suppressor, is a key regulator in energy metabolism. However, the role of LKB1 in adipogenesis is unknown. The current study aimed to determine the contributions of LKB1 to adipogenesis in vivo. Using the Fabp4-Cre/loxP system, we generated adipose tissue-specific LKB1 knockout (LKB1(ad-/-)) mice. LKB1(ad-/-) mice exhibited a reduced amount of WAT, postnatal growth retardation, and early death before weaning. Further, LKB1 deletion markedly reduced the levels of insulin receptor substrate 1 (IRS1), peroxisome proliferator-activated receptor γ, CCAAT/enhancer-binding protein α, and phosphorylated AMP-activated protein kinase (AMPK). Consistent with these results, overexpression of constitutively active AMPK partially ablated IRS1 degradation in LKB1-deficient cells. LKB1 deletion increased the levels of F-box/WD repeat-containing protein (Fbw) 8, the IRS1 ubiquitination E3 ligase. Silencing of Fbw8 increased IRS1 levels. Finally, promoter analysis and DNA chromatin immunoprecipitation analysis identified three sterol regulatory element (SRE) sites in the Fbw8 promoter, where SRE-binding protein 1c binds and induces the expression of Fbw8. Taken together, these data indicate that LKB1 controls IRS1-dependent adipogenesis via AMPK in WAT. | 23396401
 |
RIP140-targeted repression of gene expression in adipocytes.
Christian, M; Kiskinis, E; Debevec, D; Leonardsson, G; White, R; Parker, MG
Molecular and cellular biology
25
9383-91
2005
显示摘要
Ligand-dependent repression of nuclear receptor activity forms a novel mechanism for regulating gene expression. To investigate the intrinsic role of the corepressor RIP140, we have monitored gene expression profiles in cells that express or lack the RIP140 gene and that can be induced to undergo adipogenesis in vitro. In contrast to normal white adipose tissue and in vitro-differentiated wild-type adipocytes, RIP140-null cells show elevated energy expenditure and express high levels of the uncoupling protein 1 gene (Ucp1), carnitine palmitoyltransferase 1b, and the cell-death-inducing DFF45-like effector A. Conversely, all these changes are abrogated by the reexpression of RIP140. Analysis of the Ucp1 promoter showed RIP140 recruitment to a key enhancer element, demonstrating a direct role in repressing gene expression. Therefore, reduction in the levels of RIP140 or prevention of its recruitment to nuclear receptors may provide novel mechanisms for the control of energy expenditure in adipose cells. | 16227589
 |
Distribution of S-100 protein and its subunits in bovine exocrine glands.
S Lauboeck, M Egerbacher
Histochemistry and cell biology
108
83-91
1997
显示摘要
The distribution of S-100 protein and its alpha- and beta-subunits in bovine exocrine glands was studied by indirect immunohistochemistry. The entire spectrum of salivary glands, glands of the respiratory tract, intestinal glands, male and female genital glands, and skin glands was examined. S-100 and its beta-subunit were identified in most serous secretory cells of mixed salivary glands, although secretory acini in some serous glands remained unreactive for these antigens. Mucous cells were constantly negative; mucoid cells were positive in the lacrimal and Harderian gland. The alpha-subunit of S-100 protein was identified in serous cells but the staining reaction was faint. Subunits of S-100 showed a characteristic distribution along the excretory duct systems of compound glands: S-100 and the beta-subunit were present in intercalated duct epithelium, while striated duct epithelium stained for S100-alpha. Therefore, it is suggested that S100-alpha is related to resorption and secretion in striated ducts, while S100-beta may govern acinar exocytosis and probably regulates proliferation and differentiation of glandular cells. Differing staining intensities for S-100 and its subunits in secretory cells of exocrine glands most probably indicate functional differences with regard to secretory activity and the cell cycle. | 9377228
 |
The mitochondrial uncoupling protein gene. Correlation of exon structure to transmembrane domains.
Kozak, L P, et al.
J. Biol. Chem., 263: 12274-7 (1988)
1988
显示摘要
The mitochondrial uncoupling protein, a protein essential for the thermogenic properties of brown fat in mammals, is inserted in the inner mitochondrial membrane by means of six alpha-helical hydrophobic transmembrane domains. We have sequenced a complete cDNA and parts of the gene to determine that the mitochondrial uncoupling protein gene is composed of six exons, each of which encodes a transmembrane domain. We also show that transcription of the uncoupling protein gene is from a single start site; however, the use of alternative poly(A) addition signal sequences results in two mRNAs, the major species of 1221 nucleotides, not including the poly(A) tail, and a minor species of about 1600 nucleotides. The 5'-untranslated region of the mRNA is composed of 231 nucleotides, and the 3'-untranslated region contains 81 nucleotides prior to addition of the poly(A) tail. | 3410843
 |