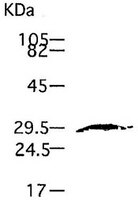IM40 Sigma-AldrichAnti-MMP-7 (Ab-1) Mouse mAb (141-7B2)
This Anti-MMP-7 (Ab-1) Mouse mAb (141-7B2) is validated for use in Immunoblotting, Paraffin Sections for the detection of MMP-7 (Ab-1).
More>> This Anti-MMP-7 (Ab-1) Mouse mAb (141-7B2) is validated for use in Immunoblotting, Paraffin Sections for the detection of MMP-7 (Ab-1). Less<<Anti-MMP-7 (Ab-1) Mouse mAb (141-7B2) MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents.
同义词: Anti-Matrilysin, Anti-Matrix Metalloproteinase 7, Anti-PUMP-1
Recommended Products
概述
| Replacement Information |
|---|
重要规格表
| Species Reactivity | Host | Antibody Type |
|---|---|---|
| H | M | Monoclonal Antibody |
价格及供货情况
| 产品目录编号 | 库存情况 | 包装 | 数量 / 包装 | 价格 | 数量 | |
|---|---|---|---|---|---|---|
| IM40-100UGCN |
|
塑胶安瓿;塑胶针药瓶 | 100 μg |
|
— |
| Product Information | |
|---|---|
| Declaration | Manufactured by Daiichi Fine Chemical Co., Ltd. Not available for sale in Japan. |
| Form | Liquid |
| Formulation | In 100 mM sodium phosphate buffer, 0.1% BSA, pH 7.0. |
| Positive control | CaR-1 or TPA treated SW620 cells |
| Preservative | ≤0.1% sodium azide |
| Quality Level | MQ100 |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| 产品目录编号 | GTIN |
| IM40-100UGCN | 04055977220599 |
Documentation
Anti-MMP-7 (Ab-1) Mouse mAb (141-7B2) MSDS
| 职位 |
|---|
Anti-MMP-7 (Ab-1) Mouse mAb (141-7B2) 分析证书
| 标题 | 批号 |
|---|---|
| IM40 |
参考
| 参考信息概述 |
|---|
| Brunner, K.L, et al. 1995. Proc. Natl. Acad. Sci. USA 92, 7362. Imai, K., et al. 1995. J. Biol. Chem. 270, 6691. Lichtinghagen, R., et al. 1995. Eur. J. Clin. Chem. Clin. Biochem. 33, 65. Nakano, A., et al. 1995. J. Neurosurg. 83, 298. Takino, T., et al. 1995. J. Biol. Chem. 270, 23013. Woessner, J.F. 1991. FASEB J. 5, 2145. Yamamoto, H., et al. 1995. J. Clin. Lab. Anal. 9, 297. Gaire, M., et al. 1994. J. Biol. Chem. 269, 2032. McDonnell, S., et al. 1994. Biochem. Soc. Trans. 22, 58. Cottam, D.W. and Rees, R.C. 1993. Intl J. Oncol. 2, 861. Stetler-Stevenson, W.G., et al. 1993. FASEB J. 7, 1434. Woessner, J.F. 1991. FASEB J. 5, 2145. Liotta, L.A. and Stetler-Stevenson, W.G. 1990. in Sem. Cancer Biol., ed. M. M. Gottesman. Vol. 1(2), 99. Woessner, J.F., Jr and Taplin, C.J. 1988. J. Biol. Chem. 263, 16918. Sellers, A. and Woessner, J.F., Jr. 1980. Biochem. J. 189, 521. |