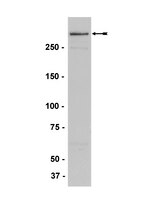
Analysis of Mll1 deficiency identifies neurogenic transcriptional modules and Brn4 as a factor for direct astrocyte-to-neuron reprogramming.
Potts, MB; Siu, JJ; Price, JD; Salinas, RD; Cho, MJ; Ramos, AD; Hahn, J; Margeta, M; Oldham, MC; Lim, DA
Neurosurgery
75
472-82; discussion 482
2014
显示摘要
Mixed lineage leukemia-1 (Mll1) epigenetically regulates gene expression patterns that specify cellular identity in both embryonic development and adult stem cell populations. In the adult mouse brain, multipotent neural stem cells (NSCs) in the subventricular zone generate new neurons throughout life, and Mll1 is required for this postnatal neurogenesis but not for glial cell differentiation. Analysis of Mll1-dependent transcription may identify neurogenic genes useful for the direct reprogramming of astrocytes into neurons.To identify Mll1-dependent transcriptional modules and to determine whether genes in the neurogenic modules can be used to directly reprogram astrocytes into neurons.We performed gene coexpression module analysis on microarray data from differentiating wild-type and Mll1-deleted subventricular zone NSCs. Key developmental regulators belonging to the neurogenic modules were overexpressed in Mll1-deleted cells and cultured cortical astrocytes, and cell phenotypes were analyzed by immunocytochemistry and electrophysiology.Transcriptional modules that correspond to neurogenesis were identified in wild-type NSCs. Modules related to astrocytes and oligodendrocytes were enriched in Mll1-deleted NSCs, consistent with their gliogenic potential. Overexpression of genes selected from the neurogenic modules enhanced the production of neurons from Mll1-deleted cells, and overexpression of Brn4 (Pou3f4) in nonneurogenic cortical astroglia induced their transdifferentiation into electrophysiologically active neurons.Our results demonstrate that Mll1 is required for the expression of neurogenic but not gliogenic transcriptional modules in a multipotent NSC population and further indicate that specific Mll1-dependent genes may be useful for direct reprogramming strategies. | | 24887289
 |
In vitro transformation of primary human CD34+ cells by AML fusion oncogenes: early gene expression profiling reveals possible drug target in AML.
Abdul-Nabi, Anmaar M, et al.
PLoS ONE, 5: e12464 (2010)
2010
显示摘要
Different fusion oncogenes in acute myeloid leukemia (AML) have distinct clinical and laboratory features suggesting different modes of malignant transformation. Here we compare the in vitro effects of representatives of 4 major groups of AML fusion oncogenes on primary human CD34+ cells. As expected from their clinical similarities, MLL-AF9 and NUP98-HOXA9 had very similar effects in vitro. They both caused erythroid hyperplasia and a clear block in erythroid and myeloid maturation. On the other hand, AML1-ETO and PML-RARA had only modest effects on myeloid and erythroid differentiation. All oncogenes except PML-RARA caused a dramatic increase in long-term proliferation and self-renewal. Gene expression profiling revealed two distinct temporal patterns of gene deregulation. Gene deregulation by MLL-AF9 and NUP98-HOXA9 peaked 3 days after transduction. In contrast, the vast majority of gene deregulation by AML1-ETO and PML-RARA occurred within 6 hours, followed by a dramatic drop in the numbers of deregulated genes. Interestingly, the p53 inhibitor MDM2 was upregulated by AML1-ETO at 6 hours. Nutlin-3, an inhibitor of the interaction between MDM2 and p53, specifically inhibited the proliferation and self-renewal of primary human CD34+ cells transduced with AML1-ETO, suggesting that MDM2 upregulation plays a role in cell transformation by AML1-ETO. These data show that differences among AML fusion oncogenes can be recapitulated in vitro using primary human CD34+ cells and that early gene expression profiling in these cells can reveal potential drug targets in AML. | | 20805992
 |
Activation of Ras-dependent Elk-1 activity by MLL-AF4 family fusion oncoproteins.
Ng, MH; Ng, RK; Kong, CT; Jin, DY; Chan, LC
Exp Hematol
38
481-8
2010
显示摘要
Mixed lineage leukemia (MLL) gene rearrangement is commonly observed in human leukemias. Many of the resultant MLL fusion proteins are found correlated with Ras signaling. Nevertheless, Ras mutations have only been reported in a small subset of MLL-rearranged leukemia. With the potential of developing new therapeutic regimens targeting Ras signaling pathway, we studied the role of MLL-AF4 family fusions and MLL-septin family fusions in the activation of Ras signaling in leukemogenesis.Elk-1-driven luciferase reporter system was used to study the role of MLL-AF4, MLL-AF5q31, MLL-LAF4, MLL-CDCrel, MLL-MSF, and MLL-Septin 6 in the activation of Ras signaling. Dominant negative Ras S17N mutant and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) inhibitor U0126 were employed to demonstrate the involvement of Ras and MEK in this transactivation event. The activation of endogenous Ras/MEK signaling pathway by MLL fusion proteins in leukemia cell lines was also addressed by immunoblot analysis and small interfering RNA knockdown approach.We demonstrated that MLL-AF4, MLL-AF5q31, and MLL-LAF4 activated Elk-1 transcription factor, one of the major downstream effectors of Ras. This activation was abolished in the presence of dominant negative Ras or MEK inhibitor U0126, indicating the requirements of Ras and MEK. We further showed that endogenous MEK is phosphorylated in a MLL-AF4-expressing leukemia cell line, whereas depletion of MLL-AF4 by small interfering RNA reduced the phospho-MEK level.Our findings suggest that MLL-AF4 family fusion oncoproteins can activate Elk-1 through Ras/MEK/extracellular signal-regulated kinase (ERK) pathway and strongly support the role of Ras signaling in the pathogenesis of MLL-rearranged leukemia. | | 20362031
 |
Crosstalk between leukemia-associated proteins MOZ and MLL regulates HOX gene expression in human cord blood CD34+ cells.
Paggetti, J, et al.
Oncogene, 29: 5019-31 (2010)
2010
显示摘要
MOZ and MLL, encoding a histone acetyltransferase (HAT) and a histone methyltransferase, respectively, are targets for recurrent chromosomal translocations found in acute myeloblastic or lymphoblastic leukemia. In MOZ (MOnocytic leukemia Zinc-finger protein)/CBP- or mixed lineage leukemia (MLL)-rearranged leukemias, abnormal levels of HOX transcription factors have been found to be critical for leukemogenesis. We show that MOZ and MLL cooperate to regulate these key genes in human cord blood CD34+ cells. These chromatin-modifying enzymes interact, colocalize and functionally cooperate, and both are recruited to multiple HOX promoters. We also found that WDR5, an adaptor protein essential for lysine 4 trimethylation of histone H3 (H3K4me3) by MLL, colocalizes and interacts with MOZ. We detected the binding of the HAT MOZ to H3K4me3, thus linking histone methylation to acetylation. In CD34+ cells, depletion of MLL causes release of MOZ from HOX promoters, which is correlated to defective histone activation marks, leading to repression of HOX gene expression and alteration of commitment of CD34+ cells into myeloid progenitors. Thus, our results unveil the role of the interaction between MOZ and MLL in CD34+ cells in which both proteins have a critical role in hematopoietic cell-fate decision, suggesting a new molecular mechanism by which MOZ or MLL deregulation leads to leukemogenesis. | | 20581860
 |
Gamma interferon-dependent transcriptional memory via relocalization of a gene locus to PML nuclear bodies.
Gialitakis, M; Arampatzi, P; Makatounakis, T; Papamatheakis, J
Molecular and cellular biology
30
2046-56
2010
显示摘要
Memory of past cellular responses is an essential adaptation to repeating environmental stimuli. We addressed the question of whether gamma interferon (IFN-gamma)-inducible transcription generates memory that sensitizes cells to a second stimulus. We have found that the major histocompatibility complex class II gene DRA is relocated to promyelocytic leukemia (PML) nuclear bodies upon induction with IFN-gamma, and this topology is maintained long after transcription shut off. Concurrent interaction of PML protein with mixed-lineage leukemia generates a prolonged permissive chromatin state on the DRA gene characterized by high promoter histone H3 K4 dimethylation that facilitates rapid expression upon restimulation. We propose that the primary signal-induced transcription generates spatial and epigenetic memory that is maintained through several cell generations and endows the cell with increased responsiveness to future activation signals. 全文本文章 | | 20123968
 |
Epigenetic regulation of the estrogen receptor alpha promoter in the cerebral cortex following ischemia in male and female rats.
Westberry, JM; Prewitt, AK; Wilson, ME
Neuroscience
152
982-9
2008
显示摘要
Permanent middle cerebral artery occlusion (MCAO) causes neuronal cell death in the striatum and cortex. In rodents, estradiol treatment protects the cortex from cell death in an estrogen receptor alpha (ERalpha) dependent manner. ERalpha is only transiently expressed in the cortex during neonatal development and is very low in uninjured adult cortex. Following MCAO, ERalpha mRNA expression is upregulated in the cortex of female rats, but the mechanism of this increase is still unknown. It is also unknown whether a similar increase in ERalpha expression in seen in males. In the following studies, male and vehicle or estradiol-treated ovariectomized (OVX) female rats underwent MCAO to investigate the regulation of ERalpha expression after ischemia. Twenty-four hours after surgery, mRNA or genomic DNA was collected from 1 mm micropunches taken from 300 mum brain sections for quantitative reverse transcription-polymerase chain reaction (RT-PCR) or methylation-specific (MSP) PCR, respectively. Additionally, adjacent 20 mum sections were processed for ERalpha immunohistochemistry. In OVX females, ERalpha mRNA and protein were increased in the ischemic cortex, but unchanged in males. We hypothesized that this increase in ERalpha in females is due to a reversal of gene silencing by DNA methylation. Using MSP targeting of CpG islands within the 5' untranslated region (UTR) of the rat ERalpha gene, we found that ischemia decreased methylation in the ischemic cortex of both groups of females, but there was no change in methylation in males. Using chromatin immunoprecipitation, we found that MeCP2 associates with ERalpha 5'UTR corresponding with the methylation status of the promoter. These data are the first to demonstrate a difference in the regulation of ERalpha expression in response to MCAO between males and females and that methylation of the ERalpha gene corresponds with mRNA levels in the brain. 全文本文章 | | 18353557
 |
MLL-AFX requires the transcriptional effector domains of AFX to transform myeloid progenitors and transdominantly interfere with forkhead protein function
So, C. W. and Cleary, M. L.
Mol Cell Biol, 22:6542-52 (2002)
2002
| Immunoprecipitation | 12192052
 |
Leukemia proto-oncoprotein MLL is proteolytically processed into 2 fragments with opposite transcriptional properties.
Yokoyama, Akihiko, et al.
Blood, 100: 3710-8 (2002)
2002
显示摘要
MLL (mixed lineage leukemia; also ALL-1 or HRX) is a proto-oncogene that is mutated in a variety of acute leukemias. Its product is normally required for the maintenance of Hox gene expression during embryogenesis and hematopoiesis through molecular mechanisms that remain poorly defined. Here we demonstrate that MLL (mixed lineage leukemia) is proteolytically processed into 2 fragments (MLL(N) and MLL(C)) that display opposite transcriptional properties and form an intramolecular MLL complex in vivo. Proteolytic cleavage occurs at 2 amino acids (D2666 and D2718) within a consensus processing sequence (QXD/GZDD, where X is a hydrophobic amino acid and Z is an alanine or a valine) that is conserved in TRX, the Drosophila homolog of MLL, and in the MLL-related protein MLL2, suggesting that processing is important for MLL function. Processed MLL(N) and MLL(C) associate with each other via N-terminal (1253-2254 amino acids) and C-terminal (3602-3742 amino acids) intramolecular interaction domains. MLL processing occurs rapidly within a few hours after translation and is followed by the phosphorylation of MLL(C). MLL(N) displays transcriptional repression activity, whereas MLL(C) has strong transcriptional activation properties. Leukemia-associated MLL fusion proteins lack the MLL processing sites, do not undergo cleavage, and are unable to interact with MLL(C). These observations suggest that posttranslational modifications of MLL may participate in regulating its activity as a transcription factor and that this aspect of its function is perturbed by leukemogenic fusions. | | 12393701
 |


















[214443-ALL].jpg)