A Site of Vulnerability on the Influenza Virus Hemagglutinin Head Domain Trimer Interface
Sandhya Bangaru 1 , Shanshan Lang 2 , Michael Schotsaert 3 , Hillary A Vanderven 4 , Xueyong Zhu 2 , Nurgun Kose 5 , Robin Bombardi 5 , Jessica A Finn 1 , Stephen J Kent 4 , Pavlo Gilchuk 5 , Iuliia Gilchuk 5 , Hannah L Turner 2 , Adolfo García-Sastre 6 , Sheng Li 7 , Andrew B Ward 2 , Ian A Wilson 8 , James E Crowe Jr
Cell
177(5)
1136-1152
2019
显示摘要
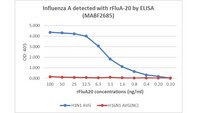
Here, we describe the discovery of a naturally occurring human antibody (Ab), FluA-20, that recognizes a new site of vulnerability on the hemagglutinin (HA) head domain and reacts with most influenza A viruses. Structural characterization of FluA-20 with H1 and H3 head domains revealed a novel epitope in the HA trimer interface, suggesting previously unrecognized dynamic features of the trimeric HA protein. The critical HA residues recognized by FluA-20 remain conserved across most subtypes of influenza A viruses, which explains the Ab's extraordinary breadth. The Ab rapidly disrupted the integrity of HA protein trimers, inhibited cell-to-cell spread of virus in culture, and protected mice against challenge with viruses of H1N1, H3N2, H5N1, or H7N9 subtypes when used as prophylaxis or therapy. The FluA-20 Ab has uncovered an exceedingly conserved protective determinant in the influenza HA head domain trimer interface that is an unexpected new target for anti-influenza therapeutics and vaccines. | 31100268
 |