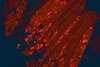
Monoallelic loss of the imprinted gene Grb10 promotes tumor formation in irradiated Nf1+/- mice.
Mroue, R; Huang, B; Braunstein, S; Firestone, AJ; Nakamura, JL
PLoS genetics
11
e1005235
2015
显示摘要
Imprinted genes are expressed from only one parental allele and heterozygous loss involving the expressed allele is sufficient to produce complete loss of protein expression. Genetic alterations are common in tumorigenesis but the role of imprinted genes in this process is not well understood. In earlier work we mutagenized mice heterozygous for the Neurofibromatosis I tumor suppressor gene (NF1) to model radiotherapy-associated second malignant neoplasms that arise in irradiated NF1 patients. Expression analysis of tumor cell lines established from our mouse models identified Grb10 expression as widely absent. Grb10 is an imprinted gene and polymorphism analysis of cell lines and primary tumors demonstrates that the expressed allele is commonly lost in diverse Nf1 mutant tumors arising in our mouse models. We performed functional studies to test whether Grb10 restoration or loss alter fundamental features of the tumor growth. Restoring Grb10 in Nf1 mutant tumors decreases proliferation, decreases soft agar colony formation and downregulates Ras signaling. Conversely, Grb10 silencing in untransformed mouse embryo fibroblasts significantly increased cell proliferation and increased Ras-GTP levels. Expression of a constitutively activated MEK rescued tumor cells from Grb10-mediated reduction in colony formation. These studies reveal that Grb10 loss can occur during in vivo tumorigenesis, with a functional consequence in untransformed primary cells. In tumors, Grb10 loss independently promotes Ras pathway hyperactivation, which promotes hyperproliferation, an early feature of tumor development. In the context of a robust Nf1 mutant mouse model of cancer this work identifies a novel role for an imprinted gene in tumorigenesis. | | 26000738
 |
MicroRNA-5p and -3p co-expression and cross-targeting in colon cancer cells.
Choo, KB; Soon, YL; Nguyen, PN; Hiew, MS; Huang, CJ
Journal of biomedical science
21
95
2014
显示摘要
Two mature miRNA species may be generated from the 5' and 3' arms of a pre-miRNA precursor. In most cases, only one species remains while the complementary species is degraded. However, co-existence of miRNA-5p and -3p species is increasingly being reported. In this work, we aimed to systematically investigate co-expression of miRNA-5p/3p in colon cancer cells in a genome-wide analysis, and to examine cross-targeting of the dysregulated miRNAs and 5p/3p species.Four colon cancer cell lines were examined relative to two normal colon tissues. Of the 1,190 miRNAs analyzed, 92 and 36 were found to be up- or down-regulated, respectively, in cancer cells. Nineteen co-expressed miRNA-5p/3p pairs were further identified suggesting frequent 5p/3p co-accumulation in colon cancer cells. Of these, 14 pairs were co-up-regulated and 3 pairs were co-down-regulated indicating concerted 5p/3p dysregulation. Nine dysregulated miRNA pairs fell into three miRNA gene families, namely let-7, mir-8/200 and mir-17, which showed frequent cross-targeting in the metastasis process. Focusing on the let-7d-5p/3p pair, the respectively targeted IGF1R and KRAS were shown to be in a reverse relationship with expression of the respective miRNA, which was confirmed in transient transfection assays using let-7d mimic or inhibitor. Targeting of KRAS by let-7d was previous reported; targeting of IGF1R by let-7d-5p was confirmed in luciferase assays in this study. The findings of let-7d-5p/3p and multiple other miRNAs targeting IGF1R, KRAS and other metastasis-related factors suggest that 5p/3p miRNAs contribute to cross-targeting of multiple cancer-associated factors and processes possibly to evade functional abolishment when any one of the crucial factors are inactivated.miRNA-5p/3p species are frequently co-expressed and are coordinately regulated in colon cancer cells. In cancer cells, multiple cross-targeting by the miRNAs, including the co-existing 5p/3p species, frequently occurs in an apparent safe-proof scheme of miRNA regulation of important tumorigenesis processes. Further systematic analysis of co-existing miRNA-5p/3p pairs in clinical tissues is important in elucidating 5p/3p contributions to cancer pathogenesis. | Western Blotting | 25287248
 |
Regulation of insulin-like growth factor I receptors in diabetic mesangial cells.
Oemar, B S, et al.
J. Biol. Chem., 266: 2369-73 (1991)
1991
显示摘要
Mesangial cells are thought to play a central role in the renal complications of diabetes mellitus. Insulin-like growth factor I (IGF-I) has been found to promote mesangial cell proliferation and regulate normal mesangial cell function in an autocrine and/or paracrine fashion. To gain further insight into the potential regulatory role IGF-I may play in mesangial cell function in diabetes, IGF-I receptors were analyzed in mesangial cells isolated from diabetic mice (db/db) and their control littermates (db/m). Mesangial cells isolated from db/db mice exhibited higher levels of IGF-I receptors compared to cells from db/m mice. Insulin receptors were not detectable in either cell type by binding analyses; however, immunoblot analysis revealed insulin receptor alpha-subunits in wheat germ agglutinin-Sepharose-purified membranes from db/db cells. Northern blot analysis further indicated a lack of detectable insulin receptor mRNA in db/m cells, whereas db/db cells expressed multiple insulin receptor mRNA transcripts. Both IGF-I and insulin receptor mRNA levels were increased in db/db cells grown in the presence of high glucose (28 mM), whereas the receptor protein levels remained relatively constant or increased, respectively. This increased expression of IGF-I and insulin receptors in diabetic mesangial cells may have an important role in the development of diabetic nephropathy. | | 1846626
 |
Identification of retinal insulin receptors using site-specific antibodies to a carboxyl-terminal peptide of the human insulin receptor alpha-subunit. Up-regulation of neuronal insulin receptors in diabetes.
Rosenzweig, S A, et al.
J. Biol. Chem., 265: 18030-4 (1990)
1990
显示摘要
Insulin receptor-specific polyclonal antipeptide serum was generated against a synthetic pentadecapeptide (residues 657-670) of the deduced amino acid sequence of human insulin proreceptor cDNA for use in the analysis of insulin receptors in the retina. The affinity-purified antibodies recognized peptide antigen but not keyhole limpet hemocyanin as determined by dot blot analysis and solid phase radioimmunoassay. Addition of either synthetic peptide or the affinity-purified serum had no effect on 125I-insulin binding to placental membranes or to cells in culture. alpha-Subunits of approximately 125 kDa from human placental membranes and liver membranes were labeled by immunoblot analysis with this antiserum. In membranes isolated from human retina and brain, two classes of alpha-subunits of approximately 125 and 115 kDa were detectable. The 115-kDa subunit was neuraminidase resistant whereas the 125-kDa subunit was digested to a band of 115 kDa, indicating that these bands represent peripheral and neuronal receptors, respectively. Analysis of human retinas obtained from type I diabetic donors revealed an increased level of neuronal receptor as compared with normal retinas. These data indicate that human retina expresses neuronal insulin receptor subtypes that are up-regulated in diabetes. | | 2211678
 |