Impact of laminitis on the canonical Wnt signaling pathway in basal epithelial cells of the equine digital laminae.
Wang, L; Pawlak, EA; Johnson, PJ; Belknap, JK; Eades, S; Stack, S; Cousin, H; Black, SJ
PloS one
8
e56025
2013
显示摘要
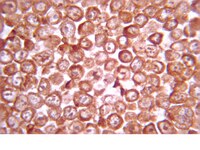
The digital laminae is a two layer tissue that attaches the distal phalanx to the inner hoof wall, thus suspending the horse's axial skeleton in the hoof capsule. This tissue fails at the epidermal:dermal junction in laminitic horses, causing crippling disease. Basal epithelial cells line the laminar epidermal:dermal junction, undergo physiological change in laminitic horses, and lose versican gene expression. Versican gene expression is purportedly under control of the canonical Wnt signaling pathway and is a trigger for mesenchymal-to-epithelial transition; thus, its repression in laminar epithelial cells of laminitic horses may be associated with suppression of the canonical Wnt signaling pathway and loss of the epithelial cell phenotype. In support of the former contention, we show, using laminae from healthy horses and horses with carbohydrate overload-induced laminitis, quantitative real-time polymerase chain reaction, Western blotting after sodium dodecylsulfate polyacrylamide gel electrophoresis, and immunofluorescent tissue staining, that positive and negative regulatory components of the canonical Wnt signaling pathway are expressed in laminar basal epithelial cells of healthy horses. Furthermore, expression of positive regulators is suppressed and negative regulators elevated in laminae of laminitic compared to healthy horses. We also show that versican gene expression in the epithelial cells correlates positively with that of β-catenin and T-cell Factor 4, consistent with regulation by the canonical Wnt signaling pathway. In addition, gene and protein expression of β-catenin correlates positively with that of integrin β4 and both are strongly suppressed in laminar basal epithelial cells of laminitic horses, which remain E-cadherin(+)/vimentin(-), excluding mesenchymal transition as contributing to loss of the adherens junction and hemidesmosome components. We propose that suppression of the canonical Wnt signaling pathway, and accompanying reduced expression of β catenin and integrin β4 in laminar basal epithelial cells reduces cell:cell and cell:basement membrane attachment, thus, destabilizing the laminar epidermal:dermal junction. | 23405249
 |
The neuron-specific isoform of glycogen synthase kinase-3beta is required for axon growth.
Castaño, Zafira, et al.
J. Neurochem., 113: 117-30 (2010)
2010
显示摘要
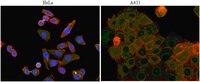
Glycogen synthase kinase-3 (GSK-3) has become an important target for the treatment of mood disorders and neurodegenerative disease. It comprises three enzymes, GSK-3alpha, beta and the neuron-specific isoform, beta2. GSK-3 regulates axon growth by phosphorylating microtubule-associated proteins including Tau. A genetic polymorphism that leads to an increase in the ratio of GSK-3beta1 to GSK-3beta2 interacts with Tau haplotypes to modify disease risk in Parkinson's and Alzheimer's disease. We have examined the roles of each isoform of GSK-3 in neurons. Silencing of GSK-3beta2 inhibited retinoic acid-induced neurite outgrowth in SH-SY5Y neuroblastoma cells and axon growth in rat cortical neurons. Inhibition of neurite outgrowth was prevented by co-expression of GSK-3beta2 but not by co-expression of GSK-3alpha or GSK-3beta1. Ectopic expression GSK-3beta2 enhanced the effects of retinoic acid on neurite length and induced neurite formation in the absence of retinoic acid. GSK-3beta2 phosphorylated Tau at a subset of those sites phosphorylated by GSK-3beta1. In addition, Axin, which regulates responses to Wnt signals, associated more readily with GSK-3beta1 than with GSK-3beta2. Our results suggest that GSK-3 inhibitors that target the Axin-binding site in GSK-3 will preserve the beneficial effects of GSK-3beta2 on axon growth. | 20067585
 |