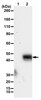
Preclinical pharmacokinetics, pharmacodynamics, and efficacy of RG7116: a novel humanized, glycoengineered anti-HER3 antibody.
Meneses-Lorente, G; Friess, T; Kolm, I; Hölzlwimmer, G; Bader, S; Meille, C; Thomas, M; Bossenmaier, B
Cancer chemotherapy and pharmacology
75
837-50
2015
显示摘要
RG7116 is a novel anti-HER3 therapeutic antibody that inhibits HER3 signalling and induces antibody-dependent cellular cytotoxicity of tumor cells due to a glycoengineered antibody Fc moiety. We investigated the efficacy and pharmacokinetic/pharmacodynamic properties of HER3 signal inhibition by RG7116 in a murine xenograft model of human head and neck cancer.SCID-beige mice bearing FaDu cells were treated with RG7116 at a weekly dose of 0.3-10 mg/kg, and tumor growth control and modulation of selected proteins (HER3 and AKT) were examined.Complete tumor stasis up to Day 46 was observed at a dose greater than 3 mg/kg, and this dose down-modulated membrane HER3 expression and inhibited HER3 and AKT phosphorylation. Systemic RG7116 exposure was greater than dose-proportional and total clearance declined with increasing dose, indicating that RG7116 elimination is target-mediated. This is consistent with the better efficacy, and the HER3 and pAKT inhibition, that was observed at doses greater than 1 mg/kg. Tumor regrowth occurred from Day 46 onwards and was associated with HER1 and HER2 upregulation, indicating the activation of alternative HER escape pathways. Modulation of HER3 and phospho-HER3 was also demonstrated in the skin and mucosa of an RG7116-treated cynomolgus monkey, suggesting that these may be useful surrogate tissues for monitoring RG7116 activity.These data confirm the promising efficacy of RG7116 and highlight the value of assessing the PK behavior of the antibody and measuring target protein modulation as a marker of biological activity. Clinical development of RG7116 has now begun, and phase I trials are ongoing. | | 25702049
 |
A leak pathway for luminal protons in endosomes drives oncogenic signalling in glioblastoma.
Kondapalli, KC; Llongueras, JP; Capilla-González, V; Prasad, H; Hack, A; Smith, C; Guerrero-Cázares, H; Quiñones-Hinojosa, A; Rao, R
Nature communications
6
6289
2015
显示摘要
Epidermal growth factor receptor (EGFR) signalling is a potent driver of glioblastoma, a malignant and lethal form of brain cancer. Disappointingly, inhibitors targeting receptor tyrosine kinase activity are not clinically effective and EGFR persists on the plasma membrane to maintain tumour growth and invasiveness. Here we show that endolysosomal pH is critical for receptor sorting and turnover. By functioning as a leak pathway for protons, the Na(+)/H(+) exchanger NHE9 limits luminal acidification to circumvent EGFR turnover and prolong downstream signalling pathways that drive tumour growth and migration. In glioblastoma, NHE9 expression is associated with stem/progenitor characteristics, radiochemoresistance, poor prognosis and invasive growth in vitro and in vivo. Silencing or inhibition of NHE9 in brain tumour-initiating cells attenuates tumoursphere formation and improves efficacy of EGFR inhibitor. Thus, NHE9 mediates inside-out control of oncogenic signalling and is a highly druggable target for pan-specific receptor clearance in cancer therapy. | | 25662504
 |
Regulation of fibroblast growth factor-inducible 14 (Fn14) expression levels via ligand-independent lysosomal degradation.
Gurunathan, S; Winkles, JA; Ghosh, S; Hayden, MS
The Journal of biological chemistry
289
12976-88
2014
显示摘要
Fibroblast growth factor-inducible 14 (Fn14) is a highly inducible cytokine receptor that engages multiple intracellular signaling pathways, including nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK). Fn14 expression is regulated by several cytokines and growth factors, and Fn14 is transiently up-regulated after injury. In contrast, in states of chronic inflammatory disease and in some solid tumors, Fn14 is persistently up-regulated. However, the post-translational regulation of Fn14 expression has not been directly investigated. Thus, we examined Fn14 proteostasis in the presence and absence of the Fn14 ligand TNF-like weak inducer of apoptosis (TWEAK). Similar to other TNF receptor superfamily members, we found that TWEAK induces Fn14 internalization and degradation. Surprisingly, we also observed rapid, TWEAK-independent, constitutive Fn14 internalization and turnover. Fn14 levels are maintained in cell culture by ongoing synthesis and trafficking of the receptor, leading to subsequent down-regulation by lysosomal degradation. Unexpectedly, the extracellular domain of Fn14 is necessary and sufficient for constitutive turnover. Based on these findings, we propose a model in which constitutive down-regulation of Fn14 facilitates dynamic regulation of Fn14 protein levels and prevents spontaneous or inappropriate receptor signaling. | | 24652288
 |
Constitutive and ligand-induced EGFR signalling triggers distinct and mutually exclusive downstream signalling networks.
Chakraborty, S; Li, L; Puliyappadamba, VT; Guo, G; Hatanpaa, KJ; Mickey, B; Souza, RF; Vo, P; Herz, J; Chen, MR; Boothman, DA; Pandita, TK; Wang, DH; Sen, GC; Habib, AA
Nature communications
5
5811
2014
显示摘要
Epidermal growth factor receptor (EGFR) overexpression plays an important oncogenic role in cancer. Regular EGFR protein levels are increased in cancer cells and the receptor then becomes constitutively active. However, downstream signals generated by constitutively activated EGFR are unknown. Here we report that the overexpressed EGFR oscillates between two distinct and mutually exclusive modes of signalling. Constitutive or non-canonical EGFR signalling activates the transcription factor IRF3 leading to expression of IFI27, IFIT1 and TRAIL. Ligand-mediated activation of EGFR switches off IRF3-dependent transcription, activates canonical extracellular signal-regulated kinase (ERK) and Akt signals, and confers sensitivity to chemotherapy and virus-induced cell death. Mechanistically, the distinct downstream signals result from a switch of EGFR-associated proteins. EGFR constitutively complexes with IRF3 and TBK1 leading to TBK1 and IRF3 phosphorylation. Addition of epidermal growth factor dissociates TBK1, IRF3 and EGFR leading to a loss of IRF3 activity, Shc-EGFR association and ERK activation. Finally, we provide evidence for non-canonical EGFR signalling in glioblastoma. | | 25503978
 |
Malignant peripheral nerve sheath tumor invasion requires aberrantly expressed EGF receptors and is variably enhanced by multiple EGF family ligands.
Byer, SJ; Brossier, NM; Peavler, LT; Eckert, JM; Watkins, S; Roth, KA; Carroll, SL
Journal of neuropathology and experimental neurology
72
219-33
2013
显示摘要
Aberrant epidermal growth factor receptor (EGFR) expression promotes the pathogenesis of malignant peripheral nerve sheath tumors (MPNSTs), the most common malignancy associated with neurofibromatosis type 1, but the mechanisms by which EGFR expression promotes MPNST pathogenesis are poorly understood. We hypothesized that inappropriately expressed EGFRs promote MPNST invasion and found that these kinases are concentrated in MPNST invadopodia in vitro. Epidermal growth factor receptor knockdown inhibited the migration of unstimulated MPNST cells in vitro, and exogenous EGF further enhanced MPNST migration in a substrate-specific manner, promoting migration on laminin and, to a lesser extent, collagen. In this setting, EGF acts as a chemotactic factor. We also found that the 7 known EGFR ligands (EGF, betacellulin, epiregulin, heparin-binding EGF, transforming growth factor-α [TGF-α], amphiregulin, and epigen) variably enhanced MPNST migration in a concentration-dependent manner, with TGF-α being particularly potent. With the exception of epigen, these factors similarly promoted the migration of nonneoplastic Schwann cells. Although transcripts encoding all 7 EGFR ligands were detected in human MPNST cells and tumor tissues, only TGF-α was consistently overexpressed and was found to colocalize with EGFR in situ. These data indicate that constitutive EGFR activation, potentially driven by autocrine or paracrine TGF-α signaling, promotes the aggressive invasive behavior characteristic of MPNSTs. | Western Blotting | 23399900
 |
Regulated intramembrane cleavage of the EGF receptor.
Hong-Jun Liao,Graham Carpenter
Traffic (Copenhagen, Denmark)
13
2012
显示摘要
Following the addition of EGF or ionomycin to A431 cells, protease activity mediates cleavage of the EGF receptor producing a 60 kDa fragment that includes the intracellular domain (ICD). This fragment is located in both membrane and nuclear fractions. On the basis of sensitivity to chemical inhibitors and overexpression of cDNAs, the rhomboid intramembrane proteases, not γ-secretase proteases, are identified as responsible for the cleavage event. Agonist-initiated cleavage occurs slowly over 3-24 h. Inhibition of calpain protease activity significantly increased the detectable level of ICD fragment. | | 22531034
 |
Epidermal growth factor receptor-targeted photosensitizer selectively inhibits EGFR signaling and induces targeted phototoxicity in ovarian cancer cells.
Abu-Yousif, AO; Moor, AC; Zheng, X; Savellano, MD; Yu, W; Selbo, PK; Hasan, T
Cancer letters
321
120-7
2012
显示摘要
Targeted photosensitizer delivery to EGFR-expressing cells was achieved in the present study using a high purity, targeted photoimmunoconjugate (PIC). When the PDT agent, benzoporphyrin derivative monoacid ring A (BPD) was coupled to an EGFR-targeting antibody (cetuximab), we observed altered cellular localization and selective phototoxicity of EGFR-positive cells, but no phototoxicity of EGFR-negative cells. Cetuximab in the PIC formulation blocked EGF-induced activation of the EGFR and downstream signaling pathways. Our results suggest that photoimmunotargeting is a useful dual strategy for the selective destruction of cancer cells and also exerts the receptor-blocking biological function of the antibody. | | 22266098
 |
Frank-ter Haar Syndrome Protein Tks4 Regulates Epidermal Growth Factor-dependent Cell Migration.
G B,Annam Gujd,Mikl Geiszt,Arp L,Anna Fekete,Szabolcs Sipeki,Julian Downward,L Buday,Gábor Bögel,Annamária Gujdár,Miklós Geiszt,Arpád Lányi,László Buday
The Journal of biological chemistry
287
2012
显示摘要
Mutations in the SH3PXD2B gene coding for the Tks4 protein are responsible for the autosomal recessive Frank-ter Haar syndrome. Tks4, a substrate of Src tyrosine kinase, is implicated in the regulation of podosome formation. Here, we report a novel role for Tks4 in the EGF signaling pathway. In EGF-treated cells, Tks4 is tyrosine-phosphorylated and associated with the activated EGF receptor. This association is not direct but requires the presence of Src tyrosine kinase. In addition, treatment of cells with LY294002, an inhibitor of PI 3-kinase, or mutations of the PX domain reduces tyrosine phosphorylation and membrane translocation of Tks4. Furthermore, a PX domain mutant (R43W) Tks4 carrying a reported point mutation in a Frank-ter Haar syndrome patient showed aberrant intracellular expression and reduced phosphoinositide binding. Finally, silencing of Tks4 was shown to markedly inhibit HeLa cell migration in a Boyden chamber assay in response to EGF or serum. Our results therefore reveal a new function for Tks4 in the regulation of growth factor-dependent cell migration. | | 22829589
 |
The role of MMP-1 in breast cancer growth and metastasis to the brain in a xenograft model.
Liu, H; Kato, Y; Erzinger, SA; Kiriakova, GM; Qian, Y; Palmieri, D; Steeg, PS; Price, JE
BMC cancer
12
583
2012
显示摘要
Brain metastasis is an increasingly common complication for breast cancer patients; approximately 15- 30% of breast cancer patients develop brain metastasis. However, relatively little is known about how these metastases form, and what phenotypes are characteristic of cells with brain metastasizing potential. In this study, we show that the targeted knockdown of MMP-1 in breast cancer cells with enhanced brain metastatic ability not only reduced primary tumor growth, but also significantly inhibited brain metastasis.Two variants of the MDA-MB-231 human breast cancer cell line selected for enhanced ability to form brain metastases in nude mice (231-BR and 231-BR3 cells) were found to express high levels of matrix metalloproteinase-1 (MMP-1). Short hairpin RNA-mediated stable knockdown of MMP-1 in 231-BR and 231-BR3 cells were established to analyze tumorigenic ability and metastatic ability.Short hairpin RNA-mediated stable knockdown of MMP-1 inhibited the invasive ability of MDA-MB 231 variant cells in vitro, and inhibited breast cancer growth when the cells were injected into the mammary fat pad of nude mice. Reduction of MMP-1 expression significantly attenuated brain metastasis and lung metastasis formation following injection of cells into the left ventricle of the heart and tail vein, respectively. There were significantly fewer proliferating cells in brain metastases of cells with reduced MMP-1 expression. Furthermore, reduced MMP-1 expression was associated with decreased TGFα release and phospho-EGFR expression in 231-BR and BR3 cells.Our results show that elevated expression of MMP-1 can promote the local growth and the formation of brain metastases by breast cancer cells. | Western Blotting | 23217186
 |
Nonmuscle Myosin II Is Required for Internalization of the Epidermal Growth Factor Receptor and Modulation of Downstream Signaling.
Jong Hyun Kim,Aibing Wang,Mary Anne Conti,Robert S Adelstein
The Journal of biological chemistry
287
2012
显示摘要
Ligand-induced internalization of the epidermal growth factor receptor (EGFR) is an important process for regulating signal transduction, cellular dynamics, and cell-cell communication. Here, we demonstrate that nonmuscle myosin II (NM II) is required for the internalization of the EGFR and to trigger the EGFR-dependent activation of ERK and AKT. The EGFR was identified as a protein that interacts with NM II by co-immunoprecipitation and mass spectrometry analysis. This interaction requires both the regulatory light chain 20 (RLC20) of NM II and the kinase domain of the EGFR. Two paralogs of NM II, NM II-A, and NM II-B can act to internalize the EGFR, depending on the cell type and paralog content of the cell line. Loss (siRNA) or inhibition (25 μm blebbistatin) of NM II attenuates the internalization of the EGFR and impairs EGFR-dependent activation of ERK and AKT. Both internalization of the EGFR and downstream signaling to ERK and AKT can be partially restored in siRNA-treated cells by introduction of wild type (WT) GFP-NM II, but cannot be restored by motor mutant NM II. Taken together, these results suggest that NM II plays a role in the internalization of the EGFR and EGFR-mediated signaling pathways. | | 22718763
 |