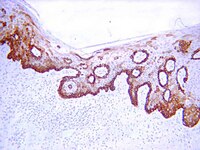
Reduced keratin expression in colorectal neoplasia and associated fields is reversible by diet and resection.
Evans, CA; Rosser, R; Waby, JS; Noirel, J; Lai, D; Wright, PC; Williams, EA; Riley, SA; Bury, JP; Corfe, BM
BMJ open gastroenterology
2
e000022
2015
显示摘要
Patients with adenomatous colonic polyps are at increased risk of developing further polyps suggesting field-wide alterations in cancer predisposition. The current study aimed to identify molecular alterations in the normal mucosa in the proximity of adenomatous polyps and to assess the modulating effect of butyrate, a chemopreventive compound produced by fermentation of dietary residues.A cross-sectional study was undertaken in patients with adenomatous polyps: biopsy samples were taken from the adenoma, and from macroscopically normal mucosa on the contralateral wall to the adenoma and from the mid-sigmoid colon. In normal subjects biopsies were taken from the mid-sigmoid colon. Biopsies were frozen for proteomic analysis or formalin-fixed for immunohistochemistry. Proteomic analysis was undertaken using iTRAQ workflows followed by bioinformatics analyses. A second dietary fibre intervention study arm used the same endpoints and sampling strategy at the beginning and end of a high-fibre intervention.Key findings were that keratins 8, 18 and 19 were reduced in expression level with progressive proximity to the lesion. Lesional tissue exhibited multiple K8 immunoreactive bands and overall reduced levels of keratin. Biopsies from normal subjects with low faecal butyrate also showed depressed keratin expression. Resection of the lesion and elevation of dietary fibre intake both appeared to restore keratin expression level.Changes in keratin expression associate with progression towards neoplasia, but remain modifiable risk factors. Dietary strategies may improve secondary chemoprevention.ISRCTN90852168. | | 26462274
 |
A Unique Expression of Keratin 14 in a Subset of Trophoblast Cells.
Abou-Kheir, W; Eid, A; El-Merahbi, R; Assaf, R; Daoud, G
PloS one
10
e0139939
2015
显示摘要
The placenta, a transient organ in human, is essential for pregnancy maintenance and for fetal growth and development. Trophoblast and stromal cells are the main cell types present in human placenta. Trophoblast cells are present in different subtypes depending on their differentiation state and their temporal and spatial location during pregnancy. The stromal cells are of extraembryonic mesenchymal origin and are important for villous formation and maintenance. Interestingly, many pregnancy-related diseases are associated with defect in trophoblast differentiation and villous integrity. Therefore, it's crucial to specifically identify each type of placental cells using specific markers. Keratins (CK) are widely used as marker of epithelial cells, cancer origin identification and in some cases as marker of stem/progenitor cells. Vimentin is widely used as marker of mesenchymal cells. The aim of this study is to characterize the presence of different keratins in human trophoblast cells and vimentin in stromal cells. Using immunohistochemistry on term placental sections, our results show that vimentin is solely expressed in stromal-mesenchymal cells while keratins 5, 7, 8, 14 and 19 are expressed in trophoblast cells. Interestingly, all keratins tested, except for keratin 14, were evenly expressed in all trophoblast cells. Keratin 14 was expressed in a subset of CK7 positive cells. Moreover, the same results were obtained when using freshly isolated cytotrophoblast cells or BeWo cells. In conclusion, this study is a crucial step in the advancement of our knowledge in placental cell type identification and characterization. | Immunofluorescence, Immunohistochemistry | 26430881
 |
High Content Imaging and Analysis Enable Quantitative In Situ Assessment of CYP3A4 Using Cryopreserved Differentiated HepaRG Cells.
Ranade, AR; Wilson, MS; McClanahan, AM; Ball, AJ
Journal of toxicology
2014
291054
2014
显示摘要
High-throughput imaging-based hepatotoxicity studies capable of analyzing individual cells in situ hold enormous promise for drug safety testing but are frequently limited by a lack of sufficient metabolically competent human cells. This study examined cryopreserved HepaRG cells, a human liver cell line which differentiates into both hepatocytes and biliary epithelial cells, to determine if these cells may represent a suitable metabolically competent cellular model for novel High Content Analysis (HCA) applications. Characterization studies showed that these cells retain many features characteristic of primary human hepatocytes and display significant CYP3A4 and CYP1A2 induction, unlike the HepG2 cell line commonly utilized for HCA studies. Furthermore, this study demonstrates that CYP3A4 induction can be quantified via a simple image analysis-based method, using HepaRG cells as a model system. Additionally, data demonstrate that the hepatocyte and biliary epithelial subpopulations characteristic of HepaRG cultures can be separated during analysis simply on the basis of nuclear size measurements. Proof of concept studies with fluorescent cell function reagents indicated that further multiparametric image-based assessment is achievable with HepaRG. In summary, image-based screening of metabolically competent human hepatocyte models cells such as HepaRG offers novel approaches for hepatotoxicity assessment and improved drug screening tools. | | 25276124
 |
The intercellular adhesion molecule, cadherin-10, is a marker for human prostate luminal epithelial cells that is not expressed in prostate cancer.
Walker, MM; Ellis, SM; Auza, MJ; Patel, A; Clark, P
Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc
21
85-95
2008
显示摘要
During the normal turnover of prostate epithelium, stem cells in the basal cell layer produce an intermediate cell population that gives rise to fully differentiated secretory luminal cells. This process is extensively studied in relation to the development of prostate disease, in particular, to elucidate the origin and nature of prostate cancer. We previously showed that the mRNA of a poorly characterised intercellular adhesion molecule, cadherin-10, is strongly expressed in human prostate. Using anticadherin-10 antibodies, immunohistochemistry, and confocal microscopy, we have examined the pattern of cadherin-10 expression in relation to human prostate epithelial differentiation markers (E-cadherin, CD44, and cytokeratins (CK) 14, 18 and 19) in archival paraffin-embedded and fixed-frozen histopathological specimens in individual and serial sections. In non-neoplastic prostate, E-cadherin is expressed by all basal and luminal epithelial cells, while cadherin-10 is variably expressed in luminal cells where it is colocalised with E-cadherin at basolateral plasma membranes. Cadherin-10 is absent in CK14- and/or CD44-positive basal cells, but is expressed in CK18-positive luminal cells (differentiated secretory cells), a subset of CK19-positive intermediate/luminal cells, but not CK19-positive basal cells. Small foci of prostate cancer express E-cadherin, CK19 and CK18, but cadherin-10 expression is low or undetectable. These findings suggest that the expression of cadherin-10 is associated with the later stages of differentiation of luminal secretory cells, indicating a specific role in secretory cell terminal differentiation. While prostate cancer cells express secretory cell markers (eg, CK18, prostate-specific antigen) and the more generally expressed E-cadherin, their failure to express cadherin-10 further emphasises a role for this cadherin in normal prostate organisation and function. | | 18084254
 |