The pre- and post-somatic segments of the human type I spiral ganglion neurons--structural and functional considerations related to cochlear implantation.
Liu, W; Edin, F; Atturo, F; Rieger, G; Löwenheim, H; Senn, P; Blumer, M; Schrott-Fischer, A; Rask-Andersen, H; Glueckert, R
Neuroscience
284
470-82
2015
显示摘要
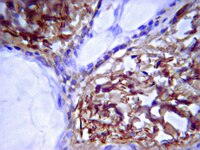
Human auditory nerve afferents consist of two separate systems; one is represented by the large type I cells innervating the inner hair cells and the other one by the small type II cells innervating the outer hair cells. Type I spiral ganglion neurons (SGNs) constitute 96% of the afferent nerve population and, in contrast to other mammals, their soma and pre- and post-somatic segments are unmyelinated. Type II nerve soma and fibers are unmyelinated. Histopathology and clinical experience imply that human SGNs can persist electrically excitable without dendrites, thus lacking connection to the organ of Corti. The biological background to this phenomenon remains elusive. We analyzed the pre- and post-somatic segments of the type I human SGNs using immunohistochemistry and transmission electron microscopy (TEM) in normal and pathological conditions. These segments were found surrounded by non-myelinated Schwann cells (NMSCs) showing strong intracellular expression of laminin-β2/collagen IV. These cells also bordered the perikaryal entry zone and disclosed surface rugosities outlined by a folded basement membrane (BM) expressing laminin-β2 and collagen IV. It is presumed that human large SGNs are demarcated by three cell categories: (a) myelinated Schwann cells, (b) NMSCs and (c) satellite glial cells (SGCs). Their BMs express laminin-β2/collagen IV and reaches the BM of the sensory epithelium at the habenula perforata. We speculate that the NMSCs protect SGNs from further degeneration following dendrite loss. It may give further explanation why SGNs can persist as electrically excitable monopolar cells even after long-time deafness, a blessing for the deaf treated with cochlear implantation. | Immunohistochemistry | | 25316409
 |
Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing.
Santos, JM; Camões, SP; Filipe, E; Cipriano, M; Barcia, RN; Filipe, M; Teixeira, M; Simões, S; Gaspar, M; Mosqueira, D; Nascimento, DS; Pinto-do-Ó, P; Cruz, P; Cruz, H; Castro, M; Miranda, JP
Stem cell research & therapy
6
90
2015
显示摘要
The secretion of trophic factors by mesenchymal stromal cells has gained increased interest given the benefits it may bring to the treatment of a variety of traumatic injuries such as skin wounds. Herein, we report on a three-dimensional culture-based method to improve the paracrine activity of a specific population of umbilical cord tissue-derived mesenchymal stromal cells (UCX®) towards the application of conditioned medium for the treatment of cutaneous wounds.A UCX® three-dimensional culture model was developed and characterized with respect to spheroid formation, cell phenotype and cell viability. The secretion by UCX® spheroids of extracellular matrix proteins and trophic factors involved in the wound-healing process was analysed. The skin regenerative potential of UCX® three-dimensional culture-derived conditioned medium (CM3D) was also assessed in vitro and in vivo against UCX® two-dimensional culture-derived conditioned medium (CM2D) using scratch and tubulogenesis assays and a rat wound splinting model, respectively.UCX® spheroids kept in our three-dimensional system remained viable and multipotent and secreted considerable amounts of vascular endothelial growth factor A, which was undetected in two-dimensional cultures, and higher amounts of matrix metalloproteinase-2, matrix metalloproteinase-9, hepatocyte growth factor, transforming growth factor β1, granulocyte-colony stimulating factor, fibroblast growth factor 2 and interleukin-6, when compared to CM2D. Furthermore, CM3D significantly enhanced elastin production and migration of keratinocytes and fibroblasts in vitro. In turn, tubulogenesis assays revealed increased capillary maturation in the presence of CM3D, as seen by a significant increase in capillary thickness and length when compared to CM2D, and increased branching points and capillary number when compared to basal medium. Finally, CM3D-treated wounds presented signs of faster and better resolution when compared to untreated and CM2D-treated wounds in vivo. Although CM2D proved to be beneficial, CM3D-treated wounds revealed a completely regenerated tissue by day 14 after excisions, with a more mature vascular system already showing glands and hair follicles.This work unravels an important alternative to the use of cells in the final formulation of advanced therapy medicinal products by providing a proof of concept that a reproducible system for the production of UCX®-conditioned medium can be used to prime a secretome for eventual clinical applications. | | | 25956381
 |
Macromolecular organization and fine structure of the human basilar membrane - RELEVANCE for cochlear implantation.
Liu, W; Atturo, F; Aldaya, R; Santi, P; Cureoglu, S; Obwegeser, S; Glueckert, R; Pfaller, K; Schrott-Fischer, A; Rask-Andersen, H
Cell and tissue research
360
245-62
2015
显示摘要
Cochlear micromechanics and frequency tuning depend on the macromolecular organization of the basilar membrane (BM), which is still unclear in man. Novel techniques in cochlear implantation (CI) motivate further analyses of the BM.Normal cochleae from patients undergoing removal of life-threatening petro-clival meningioma and an autopsy specimen from a normal human were used. Laser-confocal microscopy, high resolution scanning (SEM) and transmission electron microscopy (TEM) were carried out in combination. In addition, one human temporal bone was decellularized and investigated by SEM.The human BM consisted in four separate layers: (1) epithelial basement membrane positive for laminin-β2 and collagen IV, (2) BM "proper" composed of radial fibers expressing collagen II and XI, (3) layer of collagen IV and (4) tympanic covering layer (TCL) expressing collagen IV, fibronectin and integrin. BM thickness varied both radially and longitudinally (mean 0.55-1.16 μm). BM was thinnest near the OHC region and laterally.There are several important similarities and differences between the morphology of the BM in humans and animals. Unlike in animals, it does not contain a distinct pars tecta (arcuate) and pectinata. Its width increases and thickness decreases as it travels apically in the cochlea. Findings show that the human BM is thinnest and probably most vibration-sensitive at the outer pillar feet/Deiter cells at the OHCs. The inner pillar and IHCs seem situated on a fairly rigid part of the BM. The gradient design of the BM suggests that its vulnerability increases apical wards when performing hearing preservation CI surgery. | | | 25663274
 |
LIM kinase regulation of cytoskeletal dynamics is required for salivary gland branching morphogenesis.
Ray, S; Fanti, JA; Macedo, DP; Larsen, M
Molecular biology of the cell
25
2393-407
2014
显示摘要
Coordinated actin microfilament and microtubule dynamics is required for salivary gland development, although the mechanisms by which they contribute to branching morphogenesis are not defined. Because LIM kinase (LIMK) regulates both actin and microtubule organization, we investigated the role of LIMK signaling in mouse embryonic submandibular salivary glands using ex vivo organ cultures. Both LIMK 1 and 2 were necessary for branching morphogenesis and functioned to promote epithelial early- and late-stage cleft progression through regulation of both microfilaments and microtubules. LIMK-dependent regulation of these cytoskeletal systems was required to control focal adhesion protein-dependent fibronectin assembly and integrin β1 activation, involving the LIMK effectors cofilin and TPPP/p25, for assembly of the actin- and tubulin-based cytoskeletal systems, respectively. We demonstrate that LIMK regulates the early stages of cleft formation--cleft initiation, stabilization, and progression--via establishment of actin stability. Further, we reveal a novel role for the microtubule assembly factor p25 in regulating stabilization and elongation of late-stage progressing clefts. This study demonstrates the existence of multiple actin- and microtubule-dependent stabilization steps that are controlled by LIMK and are required in cleft progression during branching morphogenesis. | | | 24966172
 |
In vitro modeling of the neurovascular environment by coculturing adult human brain endothelial cells with human neural stem cells.
Chou, CH; Sinden, JD; Couraud, PO; Modo, M
PloS one
9
e106346
2014
显示摘要
Brain and vascular cells form a functionally integrated signalling network that is known as the neurovascular unit (NVU). The signalling (autocrine, paracrine and juxtacrine) between different elements of this unit, especially in humans, is difficult to disentangle in vivo. Developing representative in vitro models is therefore essential to better understand the cellular interactions that govern the neurovascular environment. We here describe a novel approach to assay these cellular interactions by combining a human adult cerebral microvascular endothelial cell line (hCMEC/D3) with a fetal ganglionic eminence-derived neural stem cell (hNSC) line. These cell lines provide abundant homogeneous populations of cells to produce a consistently reproducible in vitro model of endothelial morphogenesis and the ensuing NVU. Vasculature-like structures (VLS) interspersed with patches of differentiating neural cells only occurred when hNSCs were seeded onto a differentiated endothelium. These VLS emerged within 3 days of coculture and by day 6 were stabilizing. After 7 days of coculture, neuronal differentiation of hNSCs was increased 3-fold, but had no significant effect on astrocyte or oligodendrocyte differentiation. ZO1, a marker of adherens and tight junctions, was highly expressed in both undifferentiated and differentiated endothelial cells, but the adherens junction markers CD31 and VE-cadherin were significantly reduced in coculture by approximately 20%. A basement membrane, consisting of laminin, vitronectin, and collagen I and IV, separated the VLS from neural patches. This simple assay can assist in elucidating the cellular and molecular signaling involved in the formation of VLS, as well as the enhancement of neuronal differentiation through endothelial signaling. | | | 25187991
 |
Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop
Daniel Öhlund 1 , Oskar Franklin, Erik Lundberg, Christina Lundin, Malin Sund
BMC Cancer
13
154
2013
显示摘要
Background: Pancreatic cancer shows a highly aggressive and infiltrative growth pattern and is characterized by an abundant tumor stroma known to interact with the cancer cells, and to influence tumor growth and drug resistance. Cancer cells actively take part in the production of extracellular matrix proteins, which then become deposited into the tumor stroma. Type IV collagen, an important component of the basement membrane, is highly expressed by pancreatic cancer cells both in vivo and in vitro. In this study, the cellular effects of type IV collagen produced by the cancer cells were characterized. <br />Methods: The expression of type IV collagen and its integrin receptors were examined in vivo in human pancreatic cancer tissue. The cellular effects of type IV collagen were studied in pancreatic cancer cell lines by reducing type IV collagen expression through RNA interference and by functional receptor blocking of integrins and their binding-sites on the type IV collagen molecule. <br />Results: We show that type IV collagen is expressed close to the cancer cells in vivo, forming basement membrane like structures on the cancer cell surface that colocalize with the integrin receptors. Furthermore, the interaction between type IV collagen produced by the cancer cell, and integrins on the surface of the cancer cells, are important for continuous cancer cell growth, maintenance of a migratory phenotype, and for avoiding apoptosis. <br />Conclusion: We show that type IV collagen provides essential cell survival signals to the pancreatic cancer cells through an autocrine loop. | | | 23530721
 |
ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity.
Daley, WP; Gervais, EM; Centanni, SW; Gulfo, KM; Nelson, DA; Larsen, M
Development (Cambridge, England)
139
411-22
2012
显示摘要
The basement membrane is crucial for epithelial tissue organization and function. However, the mechanisms by which basement membrane is restricted to the basal periphery of epithelial tissues and the basement membrane-mediated signals that regulate coordinated tissue organization are not well defined. Here, we report that Rho kinase (ROCK) controls coordinated tissue organization by restricting basement membrane to the epithelial basal periphery in developing mouse submandibular salivary glands, and that ROCK inhibition results in accumulation of ectopic basement membrane throughout the epithelial compartment. ROCK-regulated restriction of PAR-1b (MARK2) localization in the outer basal epithelial cell layer is required for basement membrane positioning at the tissue periphery. PAR-1b is specifically required for basement membrane deposition, as inhibition of PAR-1b kinase activity prevents basement membrane deposition and disrupts overall tissue organization, and suppression of PAR-1b together with ROCK inhibition prevents interior accumulations of basement membrane. Conversely, ectopic overexpression of wild-type PAR-1b results in ectopic interior basement membrane deposition. Significantly, culture of salivary epithelial cells on exogenous basement membrane rescues epithelial organization in the presence of ROCK1 or PAR-1b inhibition, and this basement membrane-mediated rescue requires functional integrin β1 to maintain epithelial cell-cell adhesions. Taken together, these studies indicate that ROCK1/PAR-1b-dependent regulation of basement membrane placement is required for the coordination of tissue polarity and the elaboration of tissue structure in the developing submandibular salivary gland. | | | 22186730
 |
The histone methyltransferase Setd8 acts in concert with c-Myc and is required to maintain skin.
Driskell, I; Oda, H; Blanco, S; Nascimento, E; Humphreys, P; Frye, M
The EMBO journal
31
616-29
2012
显示摘要
Setd8/PR-Set7/KMT5a-dependent mono-methylation of histone H4 at lysine 20 is essential for mitosis of cultured cells; yet, the functional roles of Setd8 in complex mammalian tissues are unknown. We use skin as a model system to explore how Setd8 may regulate cell division in vivo. Deletion of Setd8 in undifferentiated layers of the mouse epidermis impaired both proliferation and differentiation processes. Long-lived epidermal progenitor cells are lost in the absence of Setd8, leading to an irreversible loss of sebaceous glands and interfollicular epidermis. We show that Setd8 is a transcriptional target of c-Myc and an essential mediator of Myc-induced epidermal differentiation. Deletion of Setd8 in c-Myc-overexpressing skin blocks proliferation and differentiation and causes apoptosis. Increased apoptosis may be explained by our discovery that p63, an essential transcription factor for epidermal commitment is lost, while p53 is gained upon removal of Setd8. Both overexpression of p63 and deletion of p53 rescue Setd8-induced apoptosis. Thus, Setd8 is a crucial inhibitor of apoptosis in skin and its activity is essential for epidermal stem cell survival, proliferation and differentiation. | | | 22117221
 |
A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis.
Daley, WP; Kohn, JM; Larsen, M
Developmental dynamics : an official publication of the American Association of Anatomists
240
2069-83
2011
显示摘要
Cleft formation is the initial step of branching morphogenesis in many organs. We previously demonstrated that ROCK 1 regulates a nonmuscle myosin II-dependent mechanochemical checkpoint to transition initiated clefts to progressing clefts in developing submandibular salivary glands. Here, we report that ROCK-mediated integrin activation and subsequent formation of focal adhesion complexes comprise this mechanochemical checkpoint. Inhibition of ROCK1 and nonmuscle myosin II activity decreased integrin β1 activation in the cleft region and interfered with localization and activation of focal adhesion complex proteins, such as focal adhesion kinase (FAK). Inhibition of FAK activity also prevented cleft progression, by disrupting recruitment of the focal adhesion proteins talin and vinculin and subsequent fibronectin assembly in the cleft region while decreasing ERK1/2 activation. These results demonstrate that inside-out integrin signaling leading to a localized recruitment of active FAK-containing focal adhesion protein complexes generates a mechanochemical checkpoint that facilitates progression of branching morphogenesis. | | | 22016182
 |
Localization of AQP5 during development of the mouse submandibular salivary gland.
Larsen, HS; Aure, MH; Peters, SB; Larsen, M; Messelt, EB; Kanli Galtung, H
Journal of molecular histology
42
71-81
2011
显示摘要
Aquaporin 5 (AQP5) is known to be central for salivary fluid secretion. A study of the temporal-spatial distribution of AQP5 during submandibular gland (SMG) development and in adult tissues might offer further clues to its unknown role during development. In the present work, SMGs from embryonic day (E) 14.5-18.5 and postnatal days (P) 0, 2, 5, 25, and 60 were immunostained for AQP5 and analyzed using light microscopy. Additional confocal and transmission electron microscopy were performed on P60 glands. Our results show that AQP5 expression first occurs in a scattered pattern in the late canalicular stage and becomes more prominent and organized in the terminal tubuli/pro-acinar cells towards birth. Additional apical membrane staining in the entire intralobular duct is found just prior to birth. During postnatal development, AQP5 is expressed in both the luminal and lateral membrane of pro-acinar/acinar cells. AQP5 is also detected in the basal membrane of acinar cells at P25 and P60. In the intercalated ducts at P60, the male glands show apical staining in the entire segment, while only the proximal region is positive in the female glands. These results demonstrate an evolving distribution of AQP5 during pre- and postnatal development in the mouse SMGs. | | | 21203896
 |