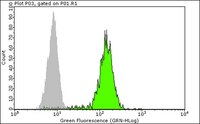
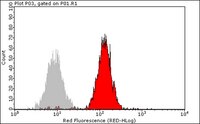
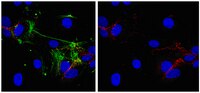
Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells.
Marlen Kolbe,Zhou Xiang,Eva Dohle,Marcus Tonak,Charles James Kirkpatrick,Sabine Fuchs
Tissue engineering. Part A
17
2011
显示摘要
Mesenchymal stem cells (MSC) from bone marrow and outgrowth endothelial cells (OEC) from peripheral blood are considered as attractive cell types for applications in regenerative medicine aiming to build up complex vascularized tissue-engineered constructs. MSC provide several advantages such as the potential to differentiate to osteoblasts and to support the neovascularization process by release of proangiogenic factors. On the other hand, the neovascularization process can be actively supported by OEC forming perfused vascular structures after co-implantation with other cell types. In this study the formation of angiogenic structures in vitro was investigated in cocultures of MSC and OEC, cultured either in the medium for osteogenic differentiation of MSC (ODM) or in the medium for OEC cultivation endothelial cell growth medium-2 (EGM2 Bullet Kit). After 2 weeks, cocultures in EGM2 formed more microvessel-like structures compared to cocultures in ODM as demonstrated by immunofluorescence staining for the endothelial marker CD31. Increased expression of CD31 and CD146 in quantitative real-time polymerase chain reaction as well as a higher percentage of CD31- and CD146-positive cells in flow cytometry indicated a beneficial influence of EGM2 on endothelial cell growth and function. In addition, the improved formation of vascular structures in EGM2 correlates with higher levels of the proangiogenic factor vascular endothelial growth factor and platelet-derived growth factor in the supernatant of cocultures as well as in monocultures of MSC when cultivated in EGM-2. Nevertheless, ODM was more suitable for the differentiation of MSC to osteoblastic lineages in the cocultures, whereas EGM2 favored factors involved in vessel stabilization by pericytes. In conclusion, this study highlights the importance of medium components for cell interaction triggering the formation of angiogenic structures. | 21529248
 |
Expression of the CD34 gene in vascular endothelial cells.
Fina, L, et al.
Blood, 75: 2417-26 (1990)
1990
显示摘要
All seven of a set of CD34 monoclonal antibodies that recognize epitopes on an approximately 110 Kd glycoprotein on human hemopoietic progenitor cells also bind to vascular endothelium. Capillaries of most tissues are CD34 positive, as are umbilical artery and, to a lesser extent, vein, but the endothelium of most large vessels and the endothelium of placental sinuses are not. Angioblastoma cells and parafollicular mesenchymal cells in fetal skin are also CD34 positive, as are some stromal elements. An approximately 110 Kd protein can be identified by Western blot analysis with CD34 antibodies in detergent extracts of freshly isolated umbilical vessel endothelial cells, and CD34 mRNA is present in cultured umbilical vein cells as well as other tissues rich in vascular endothelium (breast, placenta). These data indicate that the binding of CD34 antibodies to vascular endothelium is to the CD34 gene product, and not to crossreactive epitopes. Despite the presence of CD34 mRNA in cultured, proliferating endothelial cells, the latter do not bind CD34 antibodies. In addition, CD34 antigen cannot be upregulated by growth factors. We conclude that under these conditions, CD34 protein is downregulated or processed into another form that is unreactive with CD34 antibodies. Electron microscopy of umbilical artery, breast, and kidney capillary vessels reveals that in all three sites, CD34 molecules are concentrated on membrane processes, many of which interdigitate between adjacent endothelial cells. However, well-established endothelial cell contacts with tight junctions are CD34 negative. CD34 may function as an adhesion molecule on both endothelial cells and hematopoietic progenitors. | 1693532
 |