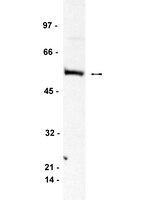
Suppression of MEK/ERK signalling by Myc: role of Bin-1.
Telfer, JF; Urquhart, J; Crouch, DH
Cellular signalling
17
701-8
2005
显示摘要
We report for the first time that over-expression of Myc suppresses mitogen-activated ERK kinase (MEK)/extracellular regulated kinase (ERK) signalling in chick embryo fibroblasts (CEF). Myc does not interfere with individual components of the signalling cascade, since efficient signal propagation via MEK and ERK in Myc-infected CEF can be seen. However, using the Myc-binding domain (MBD) of Bin-1, which binds to and negatively regulates the activity of Myc, we selectively suppressed Myc-induced apoptosis, without affecting its transforming properties. This was accompanied by a restoration in MEK/ERK signalling, suggesting a critical role for this pathway in regulating apoptosis in these cells. This was also confirmed using a specific pharmacological inhibitor of MEK. Experiments with conditioned media suggest that over-expression of Myc may inhibit autocrine growth factor production, which can be restored by co-expression of MBD. Although the identity of the growth factor(s) is not known, we propose a feedback mechanism whereby Myc interferes with growth factor signalling. | Immunohistochemistry | 15722194
 |
The putative tumor suppressor BIN1 is a short-lived nuclear phosphoprotein, the localization of which is altered in malignant cells.
Wechsler-Reya, R, et al.
Cancer Res., 57: 3258-63 (1997)
1997
显示摘要
BIN1 is a putative tumor suppressor that was identified in a genetic screen for polypeptides that interact with the MYC oncoprotein. Using a set of six monoclonal antibodies, we identified and examined biochemical features and localization of cellular BIN1. Epitope mapping indicated that a putative nuclear localization motif and the MYC-binding domain were among the regions recognized by five antibodies. In immunoprecipitation and Western analyses, cellular BIN1 was identified in human and rodent cells as a monomeric phosphoprotein of M(r) approximately 70,000. Pulse-chase experiments showed that BIN1 was short-lived, with a half-life of approximately 2 h. Cell immunofluorescence experiments revealed overlapping but unique nuclear localization patterns distinguished by two different antibodies. In normal cells, BIN1 was predominantly nucleoplasmic but was also present in a subnuclear compartment. Conversely, in a panel of tumor cells that expressed BIN1, the predominant localization was the subnuclear compartment. Taken together, the results suggested that the antibodies recognized different isoforms or conformations of BIN1, the localization of which varied between normal and tumor cells. This study will facilitate further analysis of the structure and regulation of BIN1 in normal and malignant cells. | | 9242458
 |
BIN1 is a novel MYC-interacting protein with features of a tumour suppressor.
Sakamuro, D, et al.
Nat. Genet., 14: 69-77 (1996)
1996
显示摘要
BIN1 is a novel protein that interacts with the functionally critical Myc box regions at the N terminus of the MYC oncoprotein. BIN1 is structurally related to amphiphysin, a breast cancer-associated autoimmune antigen, and RVS167, a negative regulator of the yeast cell cycle, suggesting roles in malignancy and cell cycle control. Consistent with this likelihood, BIN1 inhibited malignant cell transformation by MYC. Although BIN1 is expressed in many normal cells, its levels were greatly reduced or undetectable in 14/27 carcinoma cell lines and 3/6 primary breast tumours. Deficits were functionally significant because ectopic expression of BIN1 inhibited the growth of tumour cells lacking endogenous message. We conclude that BIN1 is an MYC-interacting protein with features of a tumour suppressor. | | 8782822
 |