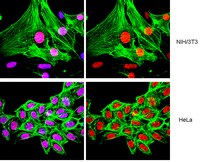
MCP-1 promotes mural cell recruitment during angiogenesis in the aortic ring model.
Alfred C Aplin,Eric Fogel,Roberto F Nicosia
Angiogenesis
13
2010
显示摘要
Rings of rat or mouse aorta embedded in collagen gels produce angiogenic outgrowths in response to the injury of the dissection procedure. Aortic outgrowths are composed of branching endothelial tubes and surrounding mural cells. Mural cells emerge following endothelial sprouting and gradually increase during the maturation of the neovessels. Treatment of aortic cultures with angiopoietin-1 (Ang-1), an angiogenic factor implicated in vascular maturation and remodeling, stimulates the mural cell recruitment process. Ang-1 induces expression of many cytokines and chemokines including monocyte chemotactic protein-1 (MCP-1). Inhibition of p38 MAP kinase, a signaling molecule required for mural cell recruitment, blocks Ang1-induced MCP-1 expression. Recombinant MCP-1 dose-dependently increases mural cell number while an anti-MCP-1 blocking antibody reduces it. In addition, antibody mediated neutralization of MCP-1 abrogates the stimulatory effect of Ang-1 on mural cell recruitment. Aortic rings from genetically modified mice deficient in MCP-1 or its receptor CCR2 have fewer mural cells than controls. MCP-1 deficiency also impairs the mural cell recruitment activity of Ang-1. Our studies indicate that spontaneous and Ang1-induced mural cell recruitment in the aortic ring of model of angiogenesis are in part mediated by MCP-1. These results implicate MCP-1 as one of the mediators of mural cell recruitment in the aortic ring model, and suggest that chemokine pathways may contribute to the assembly of the vessel wall during the angiogenesis response to injury. 全文本文章 | 20571857
 |
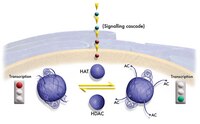
The TAF(II)250 subunit of TFIID has histone acetyltransferase activity.
Mizzen, C A, et al.
Cell, 87: 1261-70 (1996)
1996
显示摘要
The transcription initiation factor TFIID is a multimeric protein complex composed of TATA box-binding protein (TBP) and many TBP-associated factors (TAF(II)s). TAF(II)s are important cofactors that mediate activated transcription by providing interaction sites for distinct activators. Here, we present evidence that human TAF(II)250 and its homologs in Drosophila and yeast have histone acetyltransferase (HAT) activity in vitro. HAT activity maps to the central, most conserved portion of dTAF(II)230 and yTAF(II)130. The HAT activity of dTAF(II)230 resembles that of yeast and human GCN5 in that it is specific for histones H3 and H4 in vitro. Our findings suggest that targeted histone acetylation at specific promoters by TAF(II)250 may be involved in mechanisms by which TFIID gains access to transcriptionally repressed chromatin. | 8980232
 |