557366 Sigma-AldrichRosiglitazone - CAS 155141-29-0 - Calbiochem
A thiazolidinedione compound that acts as an anti-diabetic agent and serves as a potent and selective agonist of peroxisome proliferator-activated receptor-γ (PPARγ) (Kd ~40 nM) in fat cells.
More>> A thiazolidinedione compound that acts as an anti-diabetic agent and serves as a potent and selective agonist of peroxisome proliferator-activated receptor-γ (PPARγ) (Kd ~40 nM) in fat cells. Less<<Synonyms: AMPK Signaling Activator X, PPAR Agonist X, PPARγ Agonist IX, 5-[4-(2-[methyl(pyridin-2-yl)amino]ethoxy)benzyl]thiazolidine-2,4-dione, BRL49653, Avandia
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
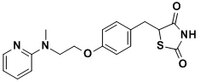
| 155141-29-0 | C₁₈H₁₉N₃O₃S.C₄H₄O₄ |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 557366-10MGCN |
|
Glass bottle | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 155141-29-0 |
| Form | White powder |
| Hill Formula | C₁₈H₁₉N₃O₃S.C₄H₄O₄ |
| Chemical formula | C₁₈H₁₉N₃O₃S.C₄H₄O₄ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥99% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 557366-10MGCN | 04055977268027 |
Documentation
Rosiglitazone - CAS 155141-29-0 - Calbiochem SDS
| Title |
|---|
Rosiglitazone - CAS 155141-29-0 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 557366 |
References
| Reference overview |
|---|
| Araki, T., et al. 2011. PPAR Res. 2011, 926438. Sozio, M. S., et al. 2011. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G739. Gerstein, H., et al. 2006. Drug 368, 9541. Mohanty, P., 2004. Journ. Clin. Endocrin. & Metab. 89(6), 2728-2735. Fryer, L., et al. 2002. J Biol Chem 277, 25226. Goldberg, R., et al. 1999. Drug. 57, 921. Young, P., et al. 1998. Journ. Pharm. Exp. Ther. 284, 751. Willson, T., et al. 1996. J. Med. Chem.39, 665; Lehmann, J., et al., 1995. JBC 270, 12953. Cantello, B., et al., 1994. Bioorg. Med. Chem. Lett. 4, 1181. |