566320 Sigma-AldrichSirtinol - CAS 410536-97-9 - Calbiochem
A cell-permeable 2-hydroxy-1-naphthaldehyde derivative that acts as a specific and direct inhibitor of the sirtuin class of deacetylase activity with no affect on human HDAC1.
More>> A cell-permeable 2-hydroxy-1-naphthaldehyde derivative that acts as a specific and direct inhibitor of the sirtuin class of deacetylase activity with no affect on human HDAC1. Less<<Synonyms: 2-[(2-Hydroxynaphthalen-1-ylmethylene)amino]-N-(1-phenethyl)benzamide, Sir Two Inhibitor Naphthol, SIRT1 Inhibitor I, SIRT1/2 Inhibitor I, SIRT2 Inhibitor III
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
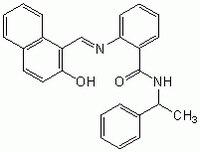
| 410536-97-9 | C₂₆H₂₂N₂O₂ |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 566320-5MG | Plastic ampoule | 5 mg |
| Description | |
|---|---|
| Overview | A cell-permeable 2-hydroxy-1-naphthaldehyde derivative that acts as a specific and direct inhibitor of the sirtuin class of deacetylase activity with no affect on human HDAC1 (IC50 = 48 µM, 131 µM and 58 µM for ySir2, hSIRT1 and hSIRT2, respectively). Reported to inhibit Sir2p transcriptional silencing activity in vivo (IC50 = 25 µM), and NAD-dependent histone deacetylase activity of purified recombinant yeast Sir2p and human SIRT2 in vitro (IC50 = 70 µM and 40 µM, respectively). A 10 mM (1 mg/254 µl) solution of Sirtinol (Cat. No. 566321) in DMSO is also available. |
| Catalogue Number | 566320 |
| Brand Family | Calbiochem® |
| Synonyms | 2-[(2-Hydroxynaphthalen-1-ylmethylene)amino]-N-(1-phenethyl)benzamide, Sir Two Inhibitor Naphthol, SIRT1 Inhibitor I, SIRT1/2 Inhibitor I, SIRT2 Inhibitor III |
| References | |
|---|---|
| References | Mai, A., et al. 2005. J. Med. Chem. 48, 7789. Grozinger, C.M., et al. 2001. J. Biol. Chem. 276, 38837. |
| Product Information | |
|---|---|
| CAS number | 410536-97-9 |
| ATP Competitive | N |
| Form | Yellow solid |
| Hill Formula | C₂₆H₂₂N₂O₂ |
| Chemical formula | C₂₆H₂₂N₂O₂ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Human HDAC1 |
| Primary Target IC<sub>50</sub> | 48 µM, 131 µM and 58 µM for ySir2, hSIRT1 and hSIRT2 |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 566320-5MG | 04055977191370 |
Documentation
Sirtinol - CAS 410536-97-9 - Calbiochem SDS
| Title |
|---|
Sirtinol - CAS 410536-97-9 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 566320 |
References
| Reference overview |
|---|
| Mai, A., et al. 2005. J. Med. Chem. 48, 7789. Grozinger, C.M., et al. 2001. J. Biol. Chem. 276, 38837. |
Data Sheet
| Title |
|---|
| SIRTainty™ Class III HDAC Assay |