382111 Sigma-AldrichHistone Acetyltransferase Inhibitor IV, CPTH2 - CAS 357649-93-5 - Calbiochem
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
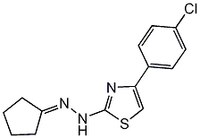
| 357649-93-5 | C₁₄H₁₄ClN₃S |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 382111-10MG | 10 mg |
| References | |
|---|---|
| References | Larrieu, D., et al. 2014. Science, 344, 527. Chimenti, F., et al. 2009. J. Med. Chem. 52, 530. |
| Product Information | |
|---|---|
| CAS number | 357649-93-5 |
| Form | Brown solid |
| Hill Formula | C₁₄H₁₄ClN₃S |
| Chemical formula | C₁₄H₁₄ClN₃S |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥95% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 382111-10MG | 04055977213102 |
Documentation
Histone Acetyltransferase Inhibitor IV, CPTH2 - CAS 357649-93-5 - Calbiochem SDS
| Title |
|---|
Histone Acetyltransferase Inhibitor IV, CPTH2 - CAS 357649-93-5 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 382111 |
References
| Reference overview |
|---|
| Larrieu, D., et al. 2014. Science, 344, 527. Chimenti, F., et al. 2009. J. Med. Chem. 52, 530. |
Technical Info
| Title |
|---|
| White Paper - The Message in the Marks: Deciphering Cancer Epigenetics |