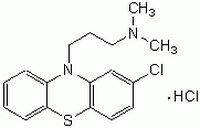
215921 Sigma-AldrichChlorpromazine, Hydrochloride - CAS 69-09-0 - Calbiochem
Inhibits calmodulin-dependent stimulation of cyclic nucleotide phosphodiesterase (IC₅₀ = 17 µM).
More>> Inhibits calmodulin-dependent stimulation of cyclic nucleotide phosphodiesterase (IC₅₀ = 17 µM). Less<<Synonyms: 2-Chloro-10-[3ʹ-(dimethylamino)propyl]phenothiazine, HCl
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 69-09-0 | C₁₇H₁₉ClN₂S · HCl |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 215921-500MG | Alu drum | 500 mg |
| Product Information | |
|---|---|
| CAS number | 69-09-0 |
| ATP Competitive | N |
| Form | Off-white crystalline solid |
| Hill Formula | C₁₇H₁₉ClN₂S · HCl |
| Chemical formula | C₁₇H₁₉ClN₂S · HCl |
| Hygroscopic | Hygroscopic |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Contaminants | Heavy metals: ≤0.002%; iron: ≤0.001%; sulfate: ≤0.05% |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | SO1750000 |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 215921-500MG | 07790788048549 |
Documentation
Chlorpromazine, Hydrochloride - CAS 69-09-0 - Calbiochem SDS
| Title |
|---|
Chlorpromazine, Hydrochloride - CAS 69-09-0 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 215921 |
References
| Reference overview |
|---|
| Lee, M.S., et al. 2007. Cancer Res. 67, 11359. Maor, I., et al. 1995. Arteriosclero. Thromb. Vasc. Biol. 15, 1378. Netea, M.G., et al. 1995. J. Infect. Dis. 171, 393. Lindahl, M., and Tagesson, C. 1993. Inflammation 17, 573. Palacios, M., et al. 1993. Biochem. Biophys. Res. Commun. 196, 280. Yamamoto, H. 1993. Toxicol. Lett. 66, 73. Vadas, P., et al. 1986. Agents Actions 19, 194. Marshak, P.R., et al. 1985. Biochemistry 24, 144. |