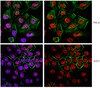
Type IV collagen α1-chain noncollagenous domain blocks MMP-2 activation both in-vitro and in-vivo.
Sudhakar, YA; Verma, RK; Pawar, SC
Scientific reports
4
4136
2014
Show Abstract
α1(IV)NC1 inhibits angiogenesis by regulating MAPK activation, this biological function was partly attributed α1(IV)NC1 binding to α1β1-integrin. However, its potent antiangiogenic activity and the molecular targets of α1(IV)NC1 has not been investigated. In the present study, the regulation of MMP-2 activation by α1(IV)NC1 was evaluated. α1β1-integrin which is required for inhibition of angiogenesis is not playing a role in cellular invasion and inhibition of MMP-2 activation by α1(IV)NC1. We found that α1(IV)NC1 binds the CBD of MMP-2 and forming a stable complex that prevents activation of MMP-2. The antiangiogenic activity of α1(IV)NC1 is mediated, in part, by this binding activity. In addition, up-regulation of TIMP-2 by α1(IV)NC1 led to saturation of MT1-MMP binding sites, which in turn led to inhibition of MMP-2 activation. In-vivo studies using α1-integrin null-mice treated with higher doses of α1(IV)NC1 showed integrin independent inhibition of tumor growth and active-MMP-2, without affecting MMP-9, MMP-7 and angiostatin. | 24670518
 |
MMP-13 stimulates osteoclast differentiation and activation in tumour breast bone metastases.
Pivetta, E; Scapolan, M; Pecolo, M; Wassermann, B; Abu-Rumeileh, I; Balestreri, L; Borsatti, E; Tripodo, C; Colombatti, A; Spessotto, P
Breast cancer research : BCR
13
R105
2011
Show Abstract
The increased bone degradation in osteolytic metastases depends on stimulation of mature osteoclasts and on continuous differentiation of new pre-osteoclasts. Metalloproteinases (MMP)-13 is expressed in a broad range of primary malignant tumours and it is emerging as a novel biomarker. Recent data suggest a direct role of MMP-13 in dissolving bone matrix complementing the activity of MMP-9 and other enzymes. Tumour-microenvironment interactions alter gene expression in malignant breast tumour cells promoting osteolytic bone metastasis. Gene expression profiles revealed that MMP-13 was among the up-regulated genes in tumour-bone interface and its abrogation reduced bone erosion. The precise mechanism remained not fully understood. Our purpose was to further investigate the mechanistic role of MMP-13 in bone osteolytic lesions.MDA-MB-231 breast cancer cells that express MMP-13 were used as a model for in vitro and in vivo experiments. Conditioned media from MDA-MB-231 cells were added to peripheral blood mononuclear cultures to monitor pre-osteoclast differentiation and activation. Bone erosion was evaluated after injection of MMP-13-silenced MDA-MB-231 cells into nude mice femurs.MMP-13 was co-expressed by human breast tumour bone metastases with its activator MT1-MMP. MMP-13 was up-regulated in breast cancer cells after in vitro stimulation with IL-8 and was responsible for increased bone resorption and osteoclastogenesis, both of which were reduced by MMP inhibitors. We hypothesized that MMP-13 might be directly involved in the loop promoting pre-osteoclast differentiation and activity. We obtained further evidence for a direct role of MMP-13 in bone metastasis by a silencing approach: conditioned media from MDA-MB-231 after MMP-13 abrogation or co-cultivation of silenced cells with pre-osteoclast were unable to increase pre-osteoclast differentiation and resorption activity. MMP-13 activated pre-MMP-9 and promoted the cleavage of galectin-3, a suppressor of osteoclastogenesis, thus contributing to pre-osteoclast differentiation. Accordingly, MMP-13 abrogation in tumour cells injected into the femurs of nude mice reduced the differentiation of TRAP positive cells in bone marrow and within the tumour mass as well as bone erosion.These results indicate that within the inflammatory bone microenvironment MMP-13 production was up-regulated in breast tumour cells leading to increased pre-osteoclast differentiation and their subsequent activation. | 22032644
 |
Integrin alpha5beta1 controls invasion of human breast carcinoma cells by direct and indirect modulation of MMP-2 collagenase activity.
Galina Morozevich,Nadezda Kozlova,Ivan Cheglakov,Natalia Ushakova,Albert Berman
Cell cycle (Georgetown, Tex.)
8
2009
Show Abstract
Integrins control a variety of signal transduction pathways central to cell survival, proliferation, and differentiation and their functions and expression levels are altered in many types of cancer. Although alpha5beta1 is one of the most studied integrins in cancer, its functions in different aspects of this disease have not been completely elucidated. In particular, controversial data exist on its role in tumor invasion and metastasis. In order to establish mechanisms underlying involvement of alpha5beta1 integrin in invasion, we depleted its expression in MCF-7Dox human breast carcinoma cells via siRNA. We demonstrated that concomitant to alpha5beta1 integrin depletion, was a sharp decrease in MMP-2 collagenase expression and inhibition of the invasiveness of these cells in vitro. Similar reduction of invasion potential was observed upon siRNA-mediated silencing of the MMP-2 gene. Down-regulation of alpha5beta1 integrin was accompanied by a substantial decrease in the amounts of active (phosphorylated) forms of Akt, Erk1/2 kinases and c-Jun oncoprotein. Moreover, in MCF-7Dox cells, blocking the activity of above kinases by specific inhibitors strongly reduced expression of MMP-2 and c-Jun, and suppressed invasion of the cells in vitro. Similar results were observed upon siRNA-mediated silencing of c-Jun expression. Co-immunoprecipitation experiments demonstrated that alpha5beta1 integrin interacts with MMP-2 collagenase on the surface of MCF-7Dox breast carcinoma and SKMel-147 human melanoma cells. Our data suggest that alpha5beta1 integrin controls invasion of the studied cells via regulation of MMP-2 collagenase expression which can occur either through signaling pathways involving PI-3K, Akt, and Erk protein kinases and the c-Jun or via direct recruitment of MMP-2 to the cell surface. | 19617714
 |
Cigarette smoke exposure inhibits extracellular MMP-2 (gelatinase A) activity in human lung fibroblasts.
La Rocca, G; Anzalone, R; Magno, F; Farina, F; Cappello, F; Zummo, G
Respiratory research
8
23
2007
Show Abstract
Exposure to cigarette smoke is considered a major risk factor for the development of lung diseases, since its causative role has been assessed in the induction and maintenance of an inflamed state in the airways. Lung fibroblasts can contribute to these processes, due to their ability to produce proinflammatory chemotactic molecules and extracellular matrix remodelling proteinases. Among proteolytic enzymes, gelatinases A and B have been studied for their role in tissue breakdown and mobilisation of matrix-derived signalling molecules. Multiple reports linked gelatinase deregulation and overexpression to the development of inflammatory chronic lung diseases such as COPD.In this study we aimed to determine variations in the gelatinolytic pattern of human lung fibroblasts (HFL-1 cell line) exposed to cigarette smoke extract (CSE). Gelatinolytic activity levels were determined by using gelatin zymography for the in-gel detection of the enzymes (proenzyme and activated forms), and the subsequent semi-quantitative densitometric evaluation of lytic bands. Expression of gelatinases was evaluated also by RT-PCR, zymography of the cell lysates and by western blotting.CSE exposure at the doses used (1-10%) did not exert any significant cytotoxic effects on fibroblasts. Zymographic analysis showed that CSE exposure resulted in a linear decrease of the activity of gelatinase A. Control experiments allowed excluding a direct inhibitory effect of CSE on gelatinases. Zymography of cell lysates confirmed the expression of MMP-2 in all conditions. Semi-quantitative evaluation of mRNA expression allowed assessing a reduced transcription of the enzyme, as well as an increase in the expression of TIMP-2. Statistical analyses showed that the decrease of MMP-2 activity in conditioned media reached the statistical significance (p = 0.0031 for 24 h and p = 0.0012 for 48 h), while correlation analysis showed that this result was independent from CSE cytotoxicity (p = 0.7833 for both exposures).Present work describes for the first time that, apart well characterized proinflammatory responses, human lung fibroblasts may react to CSE with a significant reduction of extracellular MMP-2 lytic activity. Therefore, fibroblasts may actively participate to the alteration of the proteolysis/antiproteolysis balance, which reflects the defective repair of the extracellular matrix. Such event should provide a further contribution to the maintenance of the inflamed state in the lungs. Full Text Article | 17352820
 |
Identification of Ser-386 of interferon regulatory factor 3 as critical target for inducible phosphorylation that determines activation.
Mori, Mitsuaki, et al.
J. Biol. Chem., 279: 9698-702 (2004)
2004
Show Abstract
Interferon regulatory factor (IRF)-3 is a critical transcription factor regulating innate immune responses against viral and bacterial infections. Signals activated by various pathogens are integrated by IRF-3 kinase, resulting in the specific phosphorylation of IRF-3 in the cytoplasm. This phosphorylation induces dimerization and association with the coactivators CREB-binding protein/p300, and the resultant complex activates the target genes in the nucleus. However, the phosphorylation sites that determine the active/inactive status of IRF-3 have been a source of controversy. In this study, we generated an antibody that specifically detects the phosphorylation of Ser-386 and used it as a probe. We found: 1) viral infection specifically induces phosphorylation of the Ser-386; 2) recently identified IRF-3 kinases (IKK-i/epsilon and TBK-1) phosphorylate Ser-386 and induce its dimerization; 3) phosphorylation of Ser-386 is exclusively observed with the dimer; 4) mutation at Ser-386 abolishes the dimerization potential; 5) a constitutively active 5D mutant designed to mimic phosphorylation of Ser/Thr residues other than Ser-385 and -386 is secondarily phosphorylated at Ser-386, presumably by an irrelevant kinase. These results strongly suggest that Ser-386 is the target of the IRF-3 kinase and critical determinant for the activation of IRF-3. | 14703513
 |
Matrix metalloproteinase 2 (MMP2) and MMP9 are produced by kidney collecting duct principal cells but are differentially regulated by SV40 large-T, arginine vasopressin, and epidermal growth factor.
Piedagnel, R, et al.
J. Biol. Chem., 274: 1614-20 (1999)
1999
Show Abstract
We analyzed the expression and regulation of matrix metalloproteinase 2 (MMP2) and MMP9 gelatinases in a rabbit kidney collecting duct principal cell line (RC.SVtsA58) (Prié, D., Ronco, P. M., Baudouin, B., Géniteau-Legendre, M., Antoine, M., Piedagnel, R., Estrade, S., Lelongt, B., Verroust, P. J., Cassingéna, R., and Vandewalle, A. (1991) J. Cell Biol. 113, 951-962) infected with the temperature-sensitive (ts) SV40 strain tsA58. At the permissive temperature (33 degreesC), cells produced only MMP2. Shifting cells to a nonpermissive temperature (39.5 degreesC) induced a marked increase in total gelatinolytic activity due to an increase of MMP2 and an induction of MMP9 synthesis. This effect was attributed to large-T inactivation at 39.5 degreesC because it was abolished by re-infecting the cells with wild-type SV40 strain LP. Run-on experiments showed that negative regulation of MMP2 and MMP9 by large-T was transcriptional and posttranscriptional, respectively. MMP2 and MMP9 were also produced by primary cultures of collecting duct cells. In rabbit kidney, both MMP2 and MMP9 were almost exclusively expressed in collecting duct cells, where an unexpected apical localization was observed. Arginine vasopressin and epidermal growth factor, which exert opposite hydroosmotic effects in the collecting duct, also exhibited contrasted effects on MMP9 synthesis. Epidermal growth factor increased but arginine vasopressin suppressed MMP9 at a posttranscriptional level, whereas MMP2 was not affected. These results suggest a specific physiological role of MMP2 and MMP9 in principal cells of renal collecting duct. | 9880540
 |
Rheumatoid synovial endothelial cells secrete decreased levels of tissue inhibitor of MMP (TIMP1).
Jackson, C J, et al.
Ann. Rheum. Dis., 57: 158-61 (1998)
1998
Show Abstract
OBJECTIVES: Angiogenesis (the formation of new blood vessels) is a major component of the inflammatory pannus in rheumatoid arthritis (RA). Matrix metalloproteinase (MMP) secretion by microvascular endothelial cells is an essential step in angiogenesis. The secretion of MMP1, MMP2, MMP9, and TIMP1 by human microvascular endothelial cells derived from RA synovium (RASE) to normal synovium (NSE) and neonatal foreskin (FSE) was compared. METHODS: Confluent monolayers of endothelial cells in basal medium were pre-incubated for 24 hours in the presence or absence of phorbol myristate acetate (PMA, 100 ng/ml). MMP1 activity was measured using a spectrophotometric assay and western blotting. MMP2 and MMP9 were measured using zymography. TIMP1 was measured by enzyme linked immunosorbent assay and western blotting. RESULTS: There was little difference between the amounts of MMP2 secreted by any of the cell lines. In response to PMA both synovial cell types showed a significantly higher MMP1 and MMP9 activity compared with FSE, although there was no difference between RASE and NSE. Tumour necrosis factor alpha had minimal effect on MMP activity. There was a striking decrease in the amount of TIMP1 secreted by RASE compared with normal synovium. CONCLUSIONS: As overall MMP activity is a balance between the amount of MMP and TIMP1 present, the low levels of TIMP1 produced by RASE would shift the balance in favour of increased MMP activity by these cells. This is likely to contribute to the angiogenic potential of RASE. | 9640131
 |
Matrix metalloproteinase-1 is induced by epidermal growth factor in human bladder tumour cell lines and is detectable in urine of patients with bladder tumours.
Nutt, J E, et al.
Br. J. Cancer, 78: 215-20 (1998)
1998
Show Abstract
The matrix metalloproteinases are a family of enzymes that degrade the extracellular matrix and are considered to be important in tumour invasion and metastasis. The effect of epidermal growth factor (EGF) on matrix metalloproteinase-1 (MMP1) production in two human bladder tumour cell lines, RT112 and RT4, has been investigated. In the RT112 cell line, an increase in MMP1 mRNA levels was found after a 6-h incubation with EGF, and this further increased to 20-fold that of control levels at 24- and 48-h treatment with 50 ng ml(-1) of EGF. MMP2 mRNA levels remained constant over this time period, whereas in the RT4 cells no MMP2 transcripts were detectable, but MMP1 transcripts again increased with 24- and 48-h treatment with 50 ng ml(-1) of EGF. MMP1 protein concentration in the conditioned medium from both cell lines increased with 24- and 48-h treatment of the cells and the total MMP1 was higher in the medium than the cells, demonstrating that the bladder tumour cell lines synthesize and secrete MMP1 protein after continuous stimulation with EGF. MMP1 protein was detected in urine from patients with bladder tumours, with a significant increase in concentration with increased stage and grade of tumour. MMP1 urine concentrations may therefore be a useful prognostic indicator for bladder tumour progression. | 9683296
 |
Expression of invasion markers CD44v6/v3, NM23 and MMP2 in laryngeal and hypopharyngeal carcinoma.
Répássy, G, et al.
Pathol. Oncol. Res., 4: 14-21 (1998)
1998
Show Abstract
Twelve laryngeal squamous cell carcinoma cases (7 laryngeal and 5 hypopharyngeal cancer; 15 samples) were analysed by immunohistochemistry for the expression of invasion markers CD44v6/v3, NM23 and matrix metalloproteinase, MMP2. The laryngeal epithelium showed CD44v6+/v3+/NM23-/MMP2- phenotype. When tumors were grouped into TNM categories the phenotype of the T2 and T3 tumors was similar, characterised by decreased CD44v3+ and lack of MMP2 expressions. Meanwhile the NM23 expression was more frequent in T3 tumors. In T4 stage the frequency of NM23 and MMP2 positive cases increased (5/6 and 4/6, respectively) but there was no correlation with the appearence of lymph node metastasis. Comparison of the phenotype of laryngeal and hypopharyngeal tumors, irrespective of the TNM stages, revealed characteristic differences: T2 stage laryngeal tumors showed decreased CD44v3 and occasional NM23 and MMP2 positivity, while in T3 stage these tumors were characterised by increased frequency of NM23 positivity. The phenotype of the hypopharyngeal tumors was significantly different with a high frequency of MMP2 positive cases (5/6) and NM23+/low CD44v3+ phenotype. The sharp differences in the phenotypes of laryngeal and hypopharyngeal carcinomas were connected to the differences in their invasive capacity unlike to the size of the tumors, since the T4 stage hypopharyngeal tumors had a significantly smaller size than laryngeal ones, even at lower stages. | 9555115
 |
Active-MMP2 in cancer cell nests of oral cancer patients: correlation with lymph node metastasis.
Kawamata, H, et al.
Int. J. Oncol., 13: 699-704 (1998)
1998
Show Abstract
We examined the gelatinolytic activity in human oral squamous-cell carcinoma tissues in order to evaluate the capability of intravasation and extravasation of cancer cells. By a microdissection-zymography, we demonstrated separately the gelatinolytic activities in cancer cell nests and stroma adjacent to the cancer cells. The gelatinolytic activities, such as pro-matrix metalloproteinase (MMP)9 and active-MMP2 in most of cancer cell nests were much higher than those of normal gingival epithelium. Moreover, the activities of active-MMP2 in cancer cell nests of metastatic cancers were significantly higher than those of non-metastatic cancers (p<0.05). These results suggest that active-MMP2 in cancer cells can be a predictive marker for metastasis formation in oral squamous-cell carcinoma patients. | 9735398
 |