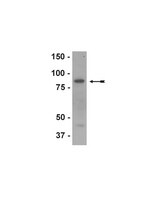
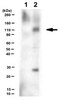
Quantification of the host response proteome after mammalian reovirus T1L infection.
Berard, AR; Cortens, JP; Krokhin, O; Wilkins, JA; Severini, A; Coombs, KM
PloS one
7
e51939
2012
Show Abstract
All viruses are dependent upon host cells for replication. Infection can induce profound changes within cells, including apoptosis, morphological changes, and activation of signaling pathways. Many of these alterations have been analyzed by gene arrays to measure the cellular "transcriptome." We used SILAC (stable isotope labeling by amino acids in cell culture), combined with high-throughput 2-D HPLC/mass spectrometry, to determine relative quantitative differences in host proteins at 6 and 24 hours after infecting HEK293 cells with reovirus serotype 1 Lang (T1L). 3,076 host proteins were detected at 6 hpi, of which 132 and 68 proteins were significantly up or down regulated, respectively. 2,992 cellular proteins, of which 104 and 49 were up or down regulated, respectively, were identified at 24 hpi. IPA and DAVID analyses indicated proteins involved in cell death, cell growth factors, oxygen transport, cell structure organization and inflammatory defense response to virus were up-regulated, whereas proteins involved in apoptosis, isomerase activity, and metabolism were down-regulated. These proteins and pathways may be suitable targets for intervention to either attenuate virus infection or enhance oncolytic potential. | Western Blotting | Human | 23240068
 |
Lactoferrin functions: current status and perspectives.
Valenti, Piera, et al.
J. Clin. Gastroenterol., 38: S127-9 (2004)
2004
Show Abstract
Lactoferrin, an iron-binding glycoprotein synthesized by neutrophils and exocrine glands, plays an important role in human innate defense mechanisms against bacteria, fungi, and viruses. First, a bacteriostatic activity of lactoferrin, depending on iron withholding to bacteria, and successively a bactericidal iron-independent effect, related to its binding on bacterial surfaces, was recognized. Many other functions have been ascribed to this cationic protein, including the inhibiting action toward bacterial adhesion and invasion of target host cells. Recent research also reported the lactoferrin influence on bacterial aggregation and biofilm development of Pseudomonas aeruginosa and Streptococcus mutans. The different lactoferrin functions can be justified by different physicochemical properties of the molecule, which include the iron-binding capability, the binding to anionic cell surfaces and molecules, and serine protease activity. | | | 15220678
 |
The antiviral activity of the milk protein lactoferrin against the human immunodeficiency virus type 1.
Berkhout, Ben, et al.
Biometals, 17: 291-4 (2004)
2004
Show Abstract
Milk forms a rich source of biologically interesting components and the protein fraction is known to facilitate many different biological functions. In this manuscript, we review the antiviral properties of the milk protein lactoferrin (LF). In particular, we will describe its antiviral activity against the human immunodeficiency virus type 1 (HIV-1). | | | 15222480
 |
Technology evaluation: rh lactoferrin, Agennix.
Andersen, Jeanette H
Curr. Opin. Mol. Ther., 6: 344-9 (2004)
2004
Show Abstract
Agennix is developing recombinant human (rh) lactoferrin, a glycoprotein with antibiotic, anti-inflammatory and immunomodulatory activity, for the potential treatment of cancers, asthma and chronic wounds. rh Lactoferrin is currently undergoing phase II clinical trials. | | | 15264438
 |
Lactoferrin--a multifunctional protein with antimicrobial properties.
Farnaud, Sebastien and Evans, Robert W
Mol. Immunol., 40: 395-405 (2003)
2003
Show Abstract
Lactoferrin is a member of the transferrin family of iron-binding proteins. Numerous functions have been reported and continue to be reported for the protein, some of which are related to its iron-binding properties. Its extensive antimicrobial activities were originally attributed to its ability to sequester essential iron, however, it is now established that it possesses bactericidal activities as a result of a direct interaction between the protein or lactoferrin-derived peptides. This article reviews the antimicrobial activities of lactoferrin and discusses the potential mode of action of lactoferrin-derived cationic peptides against Gram-negative bacteria in the light of recent studies. | | | 14568385
 |
Differential expression and estrogen response of lactoferrin gene in the female reproductive tract of mouse, rat, and hamster.
Teng, Christina T, et al.
Biol. Reprod., 67: 1439-49 (2002)
2002
Show Abstract
Lactoferrin, an iron-binding glycoprotein, kills bacteria and modulates inflammatory and immune responses. Presence of lactoferrin in the female reproductive tract suggests that the protein may be part of the mucosal immune system and act as the first line of defense against pathogenic organisms. We have discovered that lactoferrin is a major estrogen-inducible protein in the uterus of immature mice and is up-regulated by physiological levels of estrogen during proestrous in mature mice. In the present study, we examined lactoferrin gene expression and its response to estrogen stimulation in the female reproductive tract of several strains of immature mouse, rat, and hamster. The lactoferrin expression in the cycling adult female rat was also evaluated. Lactoferrin gene polymorphism exists among the different mouse strains. In the three inbred mouse strains studied, lactoferrin gene expression is stimulated by estrogen in the immature uterus, although it is less robust than in the outbred CD-1 mouse. We found that the lactoferrin gene is constitutively expressed in the epithelium of the vagina and the isthmus oviduct; however, it is estrogen inducible in the uterus of immature mice and rats. Furthermore, lactoferrin is elevated in the uterine epithelium of the mature rat during the proestrous and estrous stages of the estrous cycle. Estrogen stimulation of lactoferrin gene expression in the reproductive tract of an immature hamster is limited to the vaginal epithelium. The present study demonstrates differential expression and estrogen responsiveness of the lactoferrin gene in different regions of the female rodent reproductive tract and variation among the rodent species studied. | | | 12390874
 |
Lactoferrin gene expression is estrogen responsive in human and rhesus monkey endometrium.
Teng, Christina T, et al.
Mol. Hum. Reprod., 8: 58-67 (2002)
2002
Show Abstract
We have previously shown that the estrogen responsiveness of the human lactoferrin gene in a transient transfection system is mediated through an imperfect estrogen response element (ERE) and a steroidogenic factor 1 binding element (SFRE) 26 bp upstream from ERE. Reporter constructs containing SFRE and ERE respond to estrogen stimulation in a dose-dependent manner, whereas mutations at either one of the response elements severely impaired the estrogen responsiveness. In this study, we demonstrated that estrogen receptor (ERalpha) binds to the human lactoferrin gene ERE and forms two complexes in an electrophoresis mobility shift assay (EMSA). These complexes could be supershifted by an antibody to ERalpha. We also showed that in normal cycling women, lactoferrin gene expression in the endometrium increases during the proliferative phase and diminishes during the luteal phase. This in-vivo study thus supported the finding from transient transfection experiments that the human lactoferrin gene expression is elevated in an environment with a high level of estrogen. The estrogen effect on lactoferrin gene expression in the rhesus monkey endometrium was studied by Western blotting and immunohistochemistry. The immunohistochemistry results showed that immunoreactive lactoferrin protein was not detectable in the untreated ovariectomized monkey endometrium, was elevated by estrogen treatment, and was suppressed by sequential, combined estrogen plus progesterone treatment. In conclusion, this study has shown that lactoferrin gene expression is responsive to estrogen in primate endometrium. | | | 11756570
 |
Lactoferrin gene expression and regulation: an overview.
Teng, Christina T
Biochem. Cell Biol., 80: 7-16 (2002)
2002
Show Abstract
Lactoferrin is highly conserved among human, mouse, bovine, and porcine species. The numbers of amino acids encoded by 15 of the 17 exons in these species are identical, and in 12 locations, they have identical codon interruptions at the intron-exon splice junctions. However, lactoferrin expression is both ubiquitous and species, tissue, and cell-type specific. It is differentially regulated through multiple signaling pathways such as steroid hormone, growth factor, and kinase cascade pathways. Comparing the lactoferrin gene promoters from different species, common and different characteristics are observed. The human, mouse, bovine, porcine, and bubaline (African antelope) promoters all contain a noncanonical TATA box with an adjacent Sp1 site. Both human and mouse have multiple steroid hormone response elements, while none are found in the other species studied, suggesting that the lactoferrin gene is differentially regulated among different species by steroid hormones. Several transcription factors have been identified that are crucial for the expression of the lactoferrin gene during differentiation of the myeloid cells and in estrogen and epidermal growth factor regulation. This article provides an overview on lactoferrin expression and regulation in different species. | | | 11908645
 |
Purification and properties of an oestrogen-stimulated mouse uterine glycoprotein (approx. 70 kDa).
Teng, C T, et al.
Biochem. J., 240: 413-22 (1986)
1986
Show Abstract
An oestrogen-induced secretory protein from mouse uterine luminal fluid was purified by CM-Affi-Gel Blue chromatography and reverse-phase h.p.l.c. This protein has an apparent molecular mass of approx. 70 kDa both by SDS/polyacrylamide-gel electrophoresis (with or without 2-mercaptoethanol) and by gel-filtration column chromatography, indicating that it exists as a single-chain polypeptide. Further analysis of the protein revealed that it is highly basic (pI greater than or equal to 10) and is a glycoprotein. The N-terminus appears to be blocked to Edman degradation. The partial amino acid sequence of a fragment was obtained by cleavage with CNBr; no sequence homology was apparent between the analysed fragment and other known sequences. The incorporation of [35S]methionine into uterine proteins in vitro revealed that oestrogen treatment of immature mice stimulates both synthesis and secretion of the 70 kDa protein. An enzyme-linked immunosorbent assay with polyclonal antibody was used to determine the tissue distribution of the protein. Tissues such as lung, brain, spleen, muscle, intestine, liver, kidney and ovary of oestrogen-treated mice did not have detectable amounts of the 70 kDa protein. Immunoreactivity was present in uterine and vaginal tissues from oestrogen-treated animals. The 70 kDa protein was not induced by testosterone or progesterone. Although the function of this protein is unknown, it is useful as a marker for the study of oestrogen action in the mammalian uterus as well as regulation of gene expression at the molecular level. | | | 3814091
 |