580563 Sigma-AldrichTCPOBOP - CAS 76150-91-9 - Calbiochem
The most potent known member of the phenobarbital-like class of cytochrome P450 (CYP)-inducing agents.
More>> The most potent known member of the phenobarbital-like class of cytochrome P450 (CYP)-inducing agents. Less<<Synonymes: 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 76150-91-9 | C₁₆H₈Cl₄N₂O₂ |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 580563-25MG |
|
Ampoule plast. | 25 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 76150-91-9 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₁₆H₈Cl₄N₂O₂ |
| Chemical formula | C₁₆H₈Cl₄N₂O₂ |
| Reversible | N |
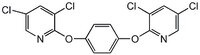
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Cytochrome P450 (CYP)-inducing agents |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | UT7145000 |
| Safety Information | |
|---|---|
| R Phrase | R: 40 Limited evidence of a carcinogenic effect. |
| S Phrase | S: 22-36 Do not breathe dust. Wear suitable protective clothing. |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 580563-25MG | 04055977265576 |
Documentation
TCPOBOP - CAS 76150-91-9 - Calbiochem FDS
| Titre |
|---|
TCPOBOP - CAS 76150-91-9 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 580563 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Ledda-Columbano, G.M., et al. 2000. Am. J. Pathol. 156, 91. Moore, L.B., et al. 2000. J. Biol. Chem. 275, 15122. Tzameli, I., et al. 2000. Mol. Cell. Biol. 20, 2951. Forman, B.M., et al. 1998. Nature 395, 612. Honkakoski, P., et al. 1998. Mol. Pharmacol. 53, 597. Russell, A.L., et al. 1994. Int. J. Cancer 58, 550. Smith, G., et al. 1993. Biochem. J. 289, 807. Dargani, T.A., et al. 1990. Carcinogenesis 11, 1153. Poland, A., et al. 1980. Mol. Pharmacol. 18, 571. |