480418 Sigma-AldrichNF279 - CAS 202983-32-2 - Calbiochem
A suramin analog that acts as a highly selective, competitive, and reversible ATP-antagonist of P2X receptor (IC₅₀/KB ~ 1 µM in smooth muscle).
More>> A suramin analog that acts as a highly selective, competitive, and reversible ATP-antagonist of P2X receptor (IC₅₀/KB ~ 1 µM in smooth muscle). Less<<Synonymes: 8,8ʹ-(Carbonylbis(imino-4,1-phenylenecarbonylimino-4,1-phenylenecarbonylimino))bis-1,3,5-naphthalenetrisulfonic Acid, Na
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
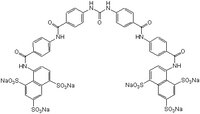
| 202983-32-2 | C₄₉H₃₀N₆O₂₃S₆ · 6Na |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 480418-5MG |
|
Ampoule plast. | 5 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 202983-32-2 |
| ATP Competitive | Y |
| Form | White solid |
| Hill Formula | C₄₉H₃₀N₆O₂₃S₆ · 6Na |
| Chemical formula | C₄₉H₃₀N₆O₂₃S₆ · 6Na |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 480418-5MG | 04055977201352 |
Documentation
NF279 - CAS 202983-32-2 - Calbiochem FDS
| Titre |
|---|
NF279 - CAS 202983-32-2 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 480418 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Klapperstück, M., et al. 2000. Eur. J. Pharmacol. 387, 245. Rettinger, J., et al. 2000. Neuropharmacol. 39, 2044. Lambrecht, G., et al. 1999. Prog. Brain Res. 120,107. Damer, S., et al. 1998. Eur. J. Pharmacol. 350, R5. |