344096 Sigma-AldrichInSolution™ Fluvastatin, Sodium Salt - CAS 93957-55-2 - Calbiochem
A synthetic, competitive inhibitor of HMG-CoA reductase that acts as anti-hypercholesterolemic agent.
More>> A synthetic, competitive inhibitor of HMG-CoA reductase that acts as anti-hypercholesterolemic agent. Less<<Synonymes: (±)-(3Rʹ,5Sʹ,6E)-7-(3-(4-Fluorophenyl)-1-isopropylindol-2-yl)-3,5-dihydroxy-6-heptenoate, sodium
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 93957-55-2 | C₂₄H₂₅FNNaO₄ |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 344096-10MG |
|
Flacon en verre | 10 mg |
|
— |
| Description | |
|---|---|
| Overview | A synthetic, competitive inhibitor of HMG-CoA reductase that acts as anti-hypercholesterolemic agent. Unlike Lovastatin (Cat. No. 438185), Mevastatin (Cat. No. 474700), and Simvastatin (Cat. No. 567020), it does not require activation prior to use for in vitro studies. Reduces the basal MMP-1 levels in culture media of endothelial cells in a time- and dose-dependent manner. Fluvastatin is metabolized in the liver, primarily via hydroxylation of the indole ring at the 5- and 6-positions. N-Dealkylation and β-oxidation of the side-chain also occurs. The hydroxy metabolites are also reported to have some pharmacologic activity. |
| Catalogue Number | 344096 |
| Brand Family | Calbiochem® |
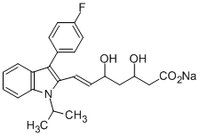
| Synonyms | (±)-(3Rʹ,5Sʹ,6E)-7-(3-(4-Fluorophenyl)-1-isopropylindol-2-yl)-3,5-dihydroxy-6-heptenoate, sodium |
| Product Information | |
|---|---|
| CAS number | 93957-55-2 |
| Form | Liquid |
| Formulation | A 10 mM (10 mg/2.13 ml) solution of Fluvastatin, Sodium Salt (344095) in H₂O. |
| Hill Formula | C₂₄H₂₅FNNaO₄ |
| Chemical formula | C₂₄H₂₅FNNaO₄ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | HMG-CoA reductase |
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 344096-10MG | 04055977215199 |
Documentation
InSolution™ Fluvastatin, Sodium Salt - CAS 93957-55-2 - Calbiochem FDS
| Titre |
|---|
InSolution™ Fluvastatin, Sodium Salt - CAS 93957-55-2 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 344096 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Yamamoto, A., et al. 2001. J. Pharm. Pharmacol. 53, 227. Dansette, P.M., et al. 2000. Exp. Toxicol. Pathol. 52, 145. Ikeda, U., et al. 2000. Hypertension 36, 325. Levy, R.I., et al. 1993. Circulation 87 (4 Suppl.), III45. Tse, F.L.S. et al. 1992. J. Clin. Pharmacol. 32, 630. Yuan, J., et al. 1991. Atherosclerosis 87, 147. |
| Fiche technique | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|