Norepinephrine is required to promote wakefulness and for hypocretin-induced arousal in zebrafish.
Singh, C; Oikonomou, G; Prober, DA
eLife
4
e07000
2015
Afficher le résumé
Pharmacological studies in mammals suggest that norepinephrine (NE) plays an important role in promoting arousal. However, the role of endogenous NE is unclear, with contradicting reports concerning the sleep phenotypes of mice lacking NE due to mutation of dopamine β-hydroxylase (dbh). To investigate NE function in an alternative vertebrate model, we generated dbh mutant zebrafish. In contrast to mice, these animals exhibit dramatically increased sleep. Surprisingly, despite an increase in sleep, dbh mutant zebrafish have a reduced arousal threshold. These phenotypes are also observed in zebrafish treated with small molecules that inhibit NE signaling, suggesting that they are caused by the lack of NE. Using genetic overexpression of hypocretin (Hcrt) and optogenetic activation of hcrt-expressing neurons, we also find that NE is important for Hcrt-induced arousal. These results establish a role for endogenous NE in promoting arousal and indicate that NE is a critical downstream effector of Hcrt neurons. | | | 26374985
 |
Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action.
Laque, A; Zhang, Y; Gettys, S; Nguyen, TA; Bui, K; Morrison, CD; Münzberg, H
American journal of physiology. Endocrinology and metabolism
304
E999-1011
2013
Afficher le résumé
Leptin acts centrally via leptin receptor (LepRb)-expressing neurons to regulate food intake, energy expenditure, and other physiological functions. LepRb neurons are found throughout the brain, and several distinct populations contribute to energy homeostasis control. However, the function of most LepRb populations remains unknown, and their contribution to regulate energy homeostasis has not been studied. Galanin has been hypothesized to interact with the leptin signaling system, but literature investigating colocalization of LepRb and galanin has been inconsistent, which is likely due to technical difficulties to visualize both. We used reporter mice with green fluorescent protein expression from the galanin locus to recapitulate the colocalization of galanin and leptin-induced p-STAT3 as a marker for LepRb expression. Here, we report the existence of two populations of galanin-expressing LepRb neurons (Gal-LepRb neurons): in the hypothalamus overspanning the perifornical area and adjacent dorsomedial and lateral hypothalamus [collectively named extended perifornical area (exPFA)] and in the brainstem (nucleus of the solitary tract). Surprisingly, despite the known orexigenic galanin action, leptin induces galanin mRNA expression and stimulates LepRb neurons in the exPFA, thus conflicting with the expected anorexigenic leptin action. However, we confirmed that intra-exPFA leptin injections were indeed sufficient to mediate anorexic responses. Interestingly, LepRb and galanin-expressing neurons are distinct from orexin or melanin-concentrating hormone (MCH)-expressing neurons, but exPFA galanin neurons colocalized with the anorexigenic neuropeptides neurotensin and cocaine- and amphetamine-regulated transcript (CART). Based on galanin's known inhibitory function, we speculate that in exPFA Gal-LepRb neurons galanin acts inhibitory rather than orexigenic. | Immunohistochemistry | Mouse | 23482448
 |
Neuropeptide S promotes wakefulness through activation of the posterior hypothalamic histaminergic and orexinergic neurons.
P Zhao,Y F Shao,M Zhang,K Fan,X P Kong,R Wang,Y P Hou
Neuroscience
207
2011
Afficher le résumé
In spite of the initial and pivotal findings that the newly identified neuropeptide S (NPS) promotes arousal associated with locomotor and anxiolytic-like effects, the mechanisms through which NPS acts to modulate sleep-waking states remain unclear. The present study was undertaken to investigate in the rat the effects of i.c.v. injection of NPS on the EEG, sleep-wake cycle, and brain c-Fos expression. NPS at 0.1 and 1 nmol increased significantly wakefulness (W) during the first 2 h (54.7 ± 3.2 and 64.9 ± 2.1 min, respectively, vs. 41.4 ± 2.5 min seen with saline injections, P<0.01 and P<0.001), accompanied by an increase in EEG high frequency activities (14.5-60 Hz). In the meanwhile, slow wave sleep (SWS) and paradoxical sleep (PS) decreased significantly. Ex-vivo Fos immunohistochemistry in the posterior hypothalamus revealed that, as compared with saline-treated rats, NPS enhanced c-Fos expression in histaminergic neurons by 76.0% in the ventral tuberomammillary nucleus (TMN) and 57.8% in the dorsal TMN, and in orexinergic neurons by 28.2% in the perifornical nucleus (PeF), 24.3% in the dorsomedial hypothalamic nucleus (DMH), and 13.7% in the lateral hypothalamic area (LH) of the posterior hypothalamus. The NPS-induced c-Fos expression in histaminergic neurons and orexinergic neurons where NPS receptor (NPSR) mRNA is highly expressed, suggests that NPS activates histaminergic and orexinergic neurons to promote W. | | | 22300983
 |
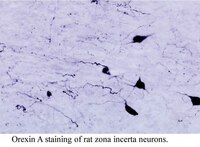
Organization and number of orexinergic neurons in the hypothalamus of two species of Cetartiodactyla: a comparison of giraffe (Giraffa camelopardalis) and harbour porpoise (Phocoena phocoena).
Dell, LA; Patzke, N; Bhagwandin, A; Bux, F; Fuxe, K; Barber, G; Siegel, JM; Manger, PR
Journal of chemical neuroanatomy
44
98-109
2011
Afficher le résumé
The present study describes the organization of the orexinergic (hypocretinergic) neurons in the hypothalamus of the giraffe and harbour porpoise--two members of the mammalian Order Cetartiodactyla which is comprised of the even-toed ungulates and the cetaceans as they share a monophyletic ancestry. Diencephalons from two sub-adult male giraffes and two adult male harbour porpoises were coronally sectioned and immunohistochemically stained for orexin-A. The staining revealed that the orexinergic neurons could be readily divided into two distinct neuronal types based on somal volume, area and length, these being the parvocellular and magnocellular orexin-A immunopositive (OxA+) groups. The magnocellular group could be further subdivided, on topological grounds, into three distinct clusters--a main cluster in the perifornical and lateral hypothalamus, a cluster associated with the zona incerta and a cluster associated with the optic tract. The parvocellular neurons were found in the medial hypothalamus, but could not be subdivided, rather they form a topologically amorphous cluster. The parvocellular cluster appears to be unique to the Cetartiodactyla as these neurons have not been described in other mammals to date, while the magnocellular nuclei appear to be homologous to similar nuclei described in other mammals. The overall size of both the parvocellular and magnocellular neurons (based on somal volume, area and length) were larger in the giraffe than the harbour porpoise, but the harbour porpoise had a higher number of both parvocellular and magnocellular orexinergic neurons than the giraffe despite both having a similar brain mass. The higher number of both parvocellular and magnocellular orexinergic neurons in the harbour porpoise may relate to the unusual sleep mechanisms in the cetaceans. | | | 22683547
 |
Orexins in the midline thalamus are involved in the expression of conditioned place aversion to morphine withdrawal.
Yonghui Li,Huiying Wang,Keke Qi,Xiaoyu Chen,Sa Li,Nan Sui,Gilbert J Kirouac
Physiology & behavior
102
2010
Afficher le résumé
Previous studies have implicated the bed nucleus of the stria terminalis, central nucleus of the amygdala and the shell of the nucleus accumbens (collectively called the extended amygdala) as playing an important role in mediating the aversive emotion associated with opioid withdrawal. The paraventricular nucleus of the thalamus (PVT) provides a very dense input to the extended amygdala, and the PVT is densely innervated by orexin neurons, which appear to be involved in producing some of the physical and emotional effects associated with morphine withdrawal. In the present study, we confirm that the PVT is densely innervated by orexin fibers, whereas the regions of the extended amygdala associated with the effects of morphine withdrawal are poorly innervated. Microinjections of the orexin-1 receptor (OX1R) antagonist SB334867 or the orexin-2 receptor (OX2R) antagonist TCSOX229 at doses of 5.0 or 15.0 microg into the PVT region did not affect the acquisition of the conditioned place aversion (CPA) nor the physical effects produced by naloxone-precipitated morphine withdrawal. In contrast, microinjections of TCSOX229 (15.0 microg) in the PVT region significantly attenuated the expression of naloxone-induced CPA while microinjections of SB334867 at the same dose had no effect. The results from these experiments indicate a role for OX2R in the PVT on the expression of CPA associated with morphine withdrawal. Orexins may mediate the aversive effects of morphine withdrawal by engaging the extended amygdala indirectly through the action of orexins on the PVT. | | | 20951152
 |
Distribution of orexinergic neurons and their terminal networks in the brains of two species of African mole rats.
Adhil Bhagwandin,Kjell Fuxe,Nigel C Bennett,Paul R Manger
Journal of chemical neuroanatomy
41
2010
Afficher le résumé
The distribution of orexinergic cell bodies and terminal networks within the brains of two species of African mole rat (Cape-dune mole rat--Bathyergus suillus and highveld mole rat--Cryptomys hottentotus) were identified using immunohistochemistry for orexin-A. The aim of the study was to investigate possible differences in the nuclear complement and terminal distribution of this system by comparing those of the mole rats to published studies of other rodents and mammals. The wild-caught mole rats used in this study live a subterranean lifestyle and are well known for their regressed visual system, which may lead to the prediction of differences in the distribution of the cell bodies and the terminal networks; however, we found that both species of mole rat displayed orexinergic nuclei limited to the hypothalamus in regions similar to those previously reported for other rodent and mammalian species. No immunoreactive neurons could be identified, in either species of mole rat within the anterior hypothalamic paraventricular nucleus, as has been reported for Murid rodents. The terminal networks, while remaining similar between the species, are more strongly expressed in the Cape-dune mole rat than in the highveld mole rat. | | | 21093582
 |
Differential distribution of melanin-concentrating hormone (MCH)- and hypocretin (Hcrt)-immunoreactive neurons projecting to the mesopontine cholinergic complex in the rat.
Eun Y Hong,Ye S Yoon,Hyun S Lee
Brain research
1424
2010
Afficher le résumé
Hypocretin (Hcrt or orexin) and melanin-concentrating hormone (MCH) containing neurons are located in the hypothalamus and are implicated in the regulation of feeding behavior, energy homeostasis, and sleep-wake cycle. MCH and Hcrt are not co-localized within the same neuron, but these neurons project widely throughout the brain, especially to brain regions regulating arousal. Recent data indicate that HCRT and MCH neurons located medially with respect to the fornix have a differential projection pattern compared to those located lateral to the fornix. To further elucidate the projection of these neurons in the present study we use retrograde tracing methods combined with double immunofluorescence to determine the differential distribution of Hcrt- and MCH-immunoreactive neurons projecting to the pedunculopontine tegmental (PPTg) or laterodorsal tegmental (LDTg) nuclei. In rats where the retrograde tracer was confined to the PPTg/LDTg we found that there were more MCH neurons projecting to these targets compared to HCRT neurons (P<0.01). When the retrograde tracer was confined to the PPTg, there were more retrogradely labeled MCH neurons lateral to the fornix compared to MCH neurons in the medial LH subdivision (P<0.05). On the average, only about 4.5% of MCH neurons versus 6.1% of HCRT neurons project to PPTg/LDTg. Thus, very few of the MCH or HCRT neurons project to these arousal populations. Although there were significantly more MCH neurons projecting to the mesopontine cholinergic arousal zone compared to the HCRT neurons, the HCRT neurons also exert an indirect influence via the tuberomammillary nucleus. Based on the present and previous (Hong and Lee, 2011) observations, we suggest that both MCH and HCRT neurons exert a potent influence on the PPTg/LDTg, which might play an important role in arousal. | | | 22015351
 |
Cellular location and major terminal networks of the orexinergic system in the brains of five microchiropteran species.
Kruger, Jean-Leigh, et al.
J. Chem. Neuroanat., 40: 256-62 (2010)
2009
Afficher le résumé
The present study describes the distribution of Orexin-A immunoreactive cell bodies and terminal networks in the brains of five microchiropteran species. Given the specialized flight and echolocation abilities of the microchiropterans it was of interest to examine if any specific differences in a generally phylogenetically homogenous neural system could be found. The orexinergic neurons have been found within the hypothalamus of all species studied, and were represented by a large cluster that spanned the anterior, dorsomedial, perifornical and lateral hypothalamic regions, with a smaller cluster extending into the region of the medial zona incerta. Evidence for orexinergic neurons in the ventrolateral hypothalamus adjacent to the optic tract was not observed in any microchiropteran species. The terminal networks of the orexinergic neurons conformed to that previously reported in a range of mammalian species, with dense terminal networks being found in the hypothalamus, cholinergic pedunculopontine and laterodorsal tegemental nuclei, the noradrenergic locus coeruleus complex, all serotonergic nuclei, the paraventricular nuclei of the epithalamus and adjacent to the habenular nuclei. Thus, apart from the lack of neurons in the ventrolateral hypothalamus, the orexinergic system of the microchiropterans appears typically mammalian. | | | 20654711
 |
How does immune challenge inhibit ingestion of palatable food? Evidence that systemic lipopolysaccharide treatment modulates key nodal points of feeding neurocircuitry.
Park, SM; Gaykema, RP; Goehler, LE
Brain, behavior, and immunity
22
1160-72
2008
Afficher le résumé
Immune challenge induces behavioral changes including reduced ingestion of palatable food. Multiple pathways likely contribute to this effect, including viscerosensory pathways controlling hypothalamic feeding circuits or by influence on "reward" circuitry previously established to control ingestive behavior. To investigate whether the effects of immune challenge may influence this network, we compared brain activation patterns in animals trained to drink a palatable sweetened milk solution and treated systemically with either the immune stimulant lipopolysaccharide (LPS) or saline. Brain sections were processed for localization of the activation marker c-Fos in neurons of regions implicated in regulation of feeding behavior. Sweetened milk ingestion was associated with increased numbers of c-Fos positive neurons in the caudal core and shell of the nucleus accumbens (NAc), the paraventricular thalamus (PVT), central nucleus of the amygdala (CEA), the basal lateral amygdala (BLA), in orexin-A containing neurons of the lateral hypothalamus (LH), and in cocaine and amphetamine regulated transcript (CART) neurons of the arcuate hypothalamus. In LPS-treated animals sweetened milk consumption was significantly reduced, as was c-Fos induction in the hypothalamic orexin-A and CART neurons, and in the BLA. In addition, induction of c-Fos in the rostral regions of the NAc, the PVT, and CEA was increased following LPS treatment, compared to controls. The findings from this study point to a network of brain regions (LH, PVT, NAc, and BLA) previously implicated in the modulation of feeding behavior, reward, and arousal that may also contribute to neural substrates involved in the reorganization of behavioral priorities that occurs during sickness. Article en texte intégral | | | 18562160
 |
Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala.
Sa Li,Gilbert J Kirouac
The Journal of comparative neurology
506
2008
Afficher le résumé
The paraventricular nucleus of the thalamus (PVT) is part of a group of midline and intralaminar thalamic nuclei implicated in arousal and attention. This study examined the connections between the PVT and the forebrain by using the retrograde tracer cholera toxin B (CTb) and the anterograde tracer biotin dextran amine (BDA). The anterior and posterior regions of the PVT were found to send a dense projection to the nucleus accumbens. The posterior PVT was also found to provide a strong projection to the lateral bed nucleus of the stria terminalis (BST), interstitial nucleus of the posterior limb of the anterior commissure (IPAC), and central nucleus of the amygdala (CeA), regions associated with the extended amygdala. In contrast, the anterior PVT was found to send a weaker projection to the extended amygdala. The basolateral nucleus of the amygdala and the medial prefrontal cortex were found to receive a relatively weak projection from the PVT, and other regions of the BST and amygdala were found to be poorly innervated by the PVT. In addition, the PVT was found to innervate regions in the extended amygdala that contained corticotropin-releasing factor (CRF) neurons, many of which were found to receive apparent contacts from PVT fibers. The projection from the PVT to the nucleus accumbens and extended amygdala places the PVT in a key anatomical position to influence adaptive behaviors as well as the physiological and neuroendocrine responses associated with these behaviors. | | | 18022956
 |