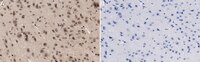
Peripheral sensitization increases opioid receptor expression and activation by crotalphine in rats.
Zambelli, VO; Fernandes, AC; Gutierrez, VP; Ferreira, JC; Parada, CA; Mochly-Rosen, D; Cury, Y
PloS one
9
e90576
2014
Afficher le résumé
Inflammation enhances the peripheral analgesic efficacy of opioid drugs, but the mechanisms involved in this phenomenon have not been fully elucidated. Crotalphine (CRP), a peptide that was first isolated from South American rattlesnake C.d. terrificus venom, induces a potent and long-lasting anti-nociceptive effect that is mediated by the activation of peripheral opioid receptors. Because the high efficacy of CRP is only observed in the presence of inflammation, we aimed to elucidate the mechanisms involved in the CRP anti-nociceptive effect induced by inflammation. Using real-time RT-PCR, western blot analysis and ELISA assays, we demonstrate that the intraplantar injection of prostaglandin E2 (PGE2) increases the mRNA and protein levels of the µ- and κ-opioid receptors in the dorsal root ganglia (DRG) and paw tissue of rats within 3 h of the injection. Using conformation state-sensitive antibodies that recognize activated opioid receptors, we show that PGE2, alone does not increase the activation of these opioid receptors but that in the presence of PGE2, the activation of specific opioid receptors by CRP and selective µ- and κ-opioid receptor agonists (positive controls) increases. Furthermore, PGE2 down-regulated the expression and activation of the δ-opioid receptor. CRP increased the level of activated mitogen-activated protein kinases in cultured DRG neurons, and this increase was dependent on the activation of protein kinase Cζ. This CRP effect was much more prominent when the cells were pretreated with PGE2. These results indicate that the expression and activation of peripheral opioid receptors by opioid-like drugs can be up- or down-regulated in the presence of an acute injury and that acute tissue injury enhances the efficacy of peripheral opioids. | Western Blotting | 24594607
 |
Peripheral δ-opioid receptors attenuate the exercise pressor reflex.
Leal, AK; Yamauchi, K; Kim, J; Ruiz-Velasco, V; Kaufman, MP
American journal of physiology. Heart and circulatory physiology
305
H1246-55
2013
Afficher le résumé
In rats with ligated femoral arteries, the exercise pressor reflex is exaggerated, an effect that is attenuated by stimulation of peripheral μ-opioid receptors on group IV metabosensitive afferents. In contrast, δ-opioid receptors are expressed mostly on group III mechanosensitive afferents, a finding that prompted us to determine whether stimulation of these opioid receptors could also attenuate the exaggerated exercise pressor reflex in "ligated" rats. We found femoral arterial injection of [D-Pen2,D-Pen5]enkephalin (DPDPE; 1.0 μg), a δ-opioid agonist, significantly attenuated the pressor and cardioaccelerator components of the exercise pressor reflex evoked by hindlimb muscle contraction in both rats with ligated and patent femoral arteries. DPDPE significantly decreased the pressor responses to muscle mechanoreflex activation, evoked by tendon stretch, in ligated rats only. DPDPE (1.0 μg) had no effect in either group on the pressor and cardioaccelerator responses to capsaicin (0.2 μg), which primarily stimulates group IV afferents. DPDPE (1.0 μg) had no effect on the pressor and cardioaccelerator responses to lactic acid (24 mM), which stimulates group III and IV afferents, in rats with patent femoral arteries but significantly decreased the pressor response in ligated rats. Western blots revealed the amount of protein comprising the δ-opioid receptor was greater in dorsal root ganglia innervating hindlimbs with ligated femoral arteries than in dorsal root ganglia innervating hindlimbs with patent femoral arteries. Our findings support the hypothesis that stimulation of δ-opioid receptors on group III afferents attenuated the exercise pressor reflex. | Western Blotting | 23934854
 |
Dopaminergic amacrine cells express opioid receptors in the mouse retina.
Gallagher, SK; Anglen, JN; Mower, JM; Vigh, J
Visual neuroscience
29
203-9
2011
Afficher le résumé
The presence of opioid receptors has been confirmed by a variety of techniques in vertebrate retinas including those of mammals; however, in most reports, the location of these receptors has been limited to retinal regions rather than specific cell types. Concurrently, our knowledge of the physiological functions of opioid signaling in the retina is based on only a handful of studies. To date, the best-documented opioid effect is the modulation of retinal dopamine release, which has been shown in a variety of vertebrate species. Nonetheless, it is not known if opioids can affect dopaminergic amacrine cells (DACs) directly, via opioid receptors expressed by DACs. This study, using immunohistochemical methods, sought to determine whether (1) μ- and δ-opioid receptors (MORs and DORs, respectively) are present in the mouse retina, and if present, (2) are they expressed by DACs. We found that MOR and DOR immunolabeling were associated with multiple cell types in the inner retina, suggesting that opioids might influence visual information processing at multiple sites within the mammalian retinal circuitry. Specifically, colabeling studies with the DAC molecular marker anti-tyrosine hydroxylase antibody showed that both MOR and DOR immunolabeling localize to DACs. These findings predict that opioids can affect DACs in the mouse retina directly, via MOR and DOR signaling, and might modulate dopamine release as reported in other mammalian and nonmammalian retinas. | Western Blotting | 22643230
 |
Disruption of morphine-conditioned place preference by a delta2-opioid receptor antagonist: study of mu-opioid and delta-opioid receptor expression at the synapse.
Billa SK, Xia Y, Morón JA
Eur J Neurosci
2009
Afficher le résumé
Abstract The addictive properties of morphine limit its clinical use. Learned associations that develop between the abused opiate and the environment in which it is consumed are engendered through Pavlovian conditioning processes. Disruption of the learned associations between the opiate and environmental cues may be a therapeutic approach to prevent morphine dependence. Although a role for the delta-opioid receptor in the regulation of the rewarding properties of morphine has already been shown, in this study we further characterized the role of the delta-opioid receptor in morphine-induced conditioned responses by examining the effect of a selective delta2-opioid receptor antagonist (naltriben), using a conditioned place preference paradigm in rats. Additionally, we used a subcellular fractionation technique to analyze the synaptic localization of mu-opioid and delta-opioid receptors in the hippocampus, in order to examine the molecular mechanisms that may underlie this morphine-induced conditioned behavior. Our data show that the administration of 1 mg/kg naltriben (but not 0.1 mg/kg) prior to morphine was able to block morphine-induced conditioned place preference. Interestingly, this naltriben-induced disruption of morphine conditioned place preference was associated with a significant increase in the expression of the delta-opioid receptor dimer at the postsynaptic density. In addition, we also observed that morphine conditioned place preference was associated with an increase in the expression of the mu-opoid receptor in the total homogenate. Overall, these results suggest that modulation of the delta-opioid receptor expression and its synaptic localization may constitute a viable therapeutic approach to disrupt morphine-induced conditioned responses. | | 20626460
 |
Nerve growth factor-regulated emergence of functional delta-opioid receptors.
Bie, B; Zhang, Z; Cai, YQ; Zhu, W; Zhang, Y; Dai, J; Lowenstein, CJ; Weinman, EJ; Pan, ZZ
The Journal of neuroscience : the official journal of the Society for Neuroscience
30
5617-28
2009
Afficher le résumé
Sorting of intracellular G-protein-coupled receptors (GPCRs) either to lysosomes for degradation or to plasma membrane for surface insertion and functional expression is a key process regulating signaling strength of GPCRs across the plasma membrane in adult mammalian cells. However, little is known about the molecular mechanisms governing the dynamic process of receptor sorting to the plasma membrane for functional expression under normal and pathological conditions. In this study, we demonstrate that delta-opioid receptor (DOPr), a GPCR constitutively targeted to intracellular compartments, is driven to the surface membrane of central synaptic terminals and becomes functional by the neurotrophin nerve growth factor (NGF) in native brainstem neurons. The NGF-triggered DOPr translocation is predominantly mediated by the signaling pathway involving the tyrosine receptor kinase A, Ca(2+)-mobilizing phospholipase C, and Ca(2+)/calmodulin-dependent protein kinase II. Importantly, it requires interactions with the cytoplasmic sorting protein NHERF-1 (Na(+)/H(+) exchange regulatory factor-1) and N-ethyl-maleimide-sensitive factor-regulated exocytosis. In addition, this NGF-mediated mechanism is likely responsible for the emergence of functional DOPr induced by chronic opioids. Thus, NGF may function as a key molecular switch that redirects the sorting of intracellularly targeted DOPr to plasma membrane, resulting in new functional DOPr on central synapses under chronic opioid conditions. Article en texte intégral | | 20410114
 |
Cell-specific actions of HIV-Tat and morphine on opioid receptor expression in glia.
Turchan-Cholewo, J; Dimayuga, FO; Ding, Q; Keller, JN; Hauser, KF; Knapp, PE; Bruce-Keller, AJ
Journal of neuroscience research
86
2100-10
2008
Afficher le résumé
HIV-1 patients who abuse opiate-based drugs, including heroin and morphine, are at a higher risk of developing HIV dementia. The effects of opiates are mediated predominantly through opioid receptors, which are expressed on glial cells. As HIV-1 infection in the CNS is restricted to glial cells, experiments were designed to measure the cell-specific effects of HIV Tat and morphine exposure on opioid receptor expression in both astrocytes and microglia. Specifically, the cell-type-specific pattern of mu opioid receptor (MOR), delta opioid receptor (DOR), and kappa opioid receptor (KOR) localization (surface vs. intracellular) and expression of opioid receptor mRNA were determined after exposure to morphine in the presence and the absence of Tat in primary cultured microglia and astrocytes. Data show that morphine treatment caused significantly decreased cell surface expression of opioid receptors in microglia but not in astrocytes. However, morphine treatment in the presence of Tat significantly increased intracellular expression of opioid receptors and prevented morphine-induced cell surface opioid receptor down-regulation in microglia. These findings document that cell surface opioid receptor expression is divergently regulated by morphine in microglia compared with in astrocytes, and further suggest that HIV-Tat could exacerbate opioid receptor signaling in microglia by increasing receptor expression and/or altering ligand-induced trafficking of opioid receptors. Article en texte intégral | | 18338799
 |
Glial-restricted precursors: patterns of expression of opioid receptors and relationship to human immunodeficiency virus-1 Tat and morphine susceptibility in vitro.
S K Buch, V K Khurdayan, S E Lutz, P E Knapp, N El-Hage, K F Hauser
Neuroscience
146
1546-54
2007
Afficher le résumé
Recent evidence suggests that human immunodeficiency virus (HIV)-induced pathogenesis is exacerbated by opioid abuse and that the synergistic toxicity may result from direct actions of opioids in immature glia or glial precursors. To assess whether opioids and HIV proteins are directly toxic to glial-restricted precursors (GRPs), we isolated neural stem cells from the incipient spinal cord of embryonic day 10.5 ICR mice. GRPs were characterized immunocytochemically and by reverse transcriptase-polymerase chain reaction (RT-PCR). At 1 day in vitro (DIV), GRPs failed to express mu opioid receptors (MOR or MOP) or kappa-opioid receptors (KOR or KOP); however, at 5 DIV, most GRPs expressed MOR and KOR. The effects of morphine (500 nM) and/or Tat (100 nM) on GRP viability were assessed in GRPs at 5 DIV by examining the apoptotic effector caspase-3 and cell viability (ethidium monoazide exclusion) at 96 h following continuous exposure. Tat or morphine alone or in combination caused significant increases in GRP cell death at 96 h, but not at 24 h, following exposure. Although morphine or Tat caused increases in caspase-3 activity at 4 h, this was not accompanied with increased cleaved caspase-3 immunoreactive or ethidium monoazide-positive dying cells at 24 h. The results indicate that prolonged morphine or Tat exposure is intrinsically toxic to isolated GRPs and/or their progeny in vitro. Moreover, MOR and KOR are widely expressed by Sox2 and/or Nkx2.2-positive GRPs in vitro and the pattern of receptor expression appears to be developmentally regulated. The temporal requirement for prolonged morphine and HIV-1 Tat exposure to evoke toxicity in glia may coincide with the attainment of a particular stage of maturation and/or the development of particular apoptotic effector pathways and may be unique to spinal cord GRPs. Should similar patterns occur in vivo then we predict that immature astroglia and oligodendroglia may be preferentially vulnerable to HIV-1 infection or chronic opiate exposure. | | 17478053
 |
Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain.
Noufissa Kabli,Catherine Marie Cahill
Pain
127
2007
Afficher le résumé
The analgesic effects of local administration of opioid agonists into peripheral tissues in alleviating pain have been well documented in both clinical and preclinical studies, although few studies have examined their effects in neuropathic pain. In this study, we investigated the anti-allodynic effects of peripherally acting delta opioid receptor (DOR) agonists in a rat model of neuropathic pain. Peripheral nerve injury (PNI) produced a time-dependent decrease in mechanical withdrawal thresholds that was attenuated by local administration into the hind paw of either morphine or the DOR agonist deltorphin II. Using Western blotting techniques, no change in DOR protein expression was detected in DRG ipsilateral to the site of injury compared to contralateral. However, an up-regulation of DOR protein was found in neuropathic DRG compared to sham, suggesting that there may be a bilateral increase in the expression of DOR following PNI. Results obtained from immunohistochemical studies confirmed up-regulation in small and large DRG neurons in neuropathic compared to sham animals. Additionally, there was an increase in DOR protein within the ipsilateral sciatic nerve of neuropathic animals compared to sham and contralateral neuropathic conditions indicating the occurrence of receptor trafficking to the site of injury. Taken together, our findings suggest that functional peripheral DORs are present in sensory neurons following PNI and validate the development of selective DOR agonists for alleviating neuropathic pain. | | 16963185
 |
Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia.
Gendron, L; Lucido, AL; Mennicken, F; O'Donnell, D; Vincent, JP; Stroh, T; Beaudet, A
The Journal of neuroscience : the official journal of the Society for Neuroscience
26
953-62
2005
Afficher le résumé
The present study demonstrates that perikaryaldelta-opioid receptors (deltaORs) in rat dorsal root ganglion (DRG) neurons bind and internalize opioid ligands circulating in the CSF. Using confocal and electron microscopy, we found that prolonged morphine treatment increased the cell surface density of these perikaryal deltaORs and, by way of consequence, receptor-mediated internalization of the fluorescent deltorphin (DLT) analog omega-Bodipy 576/589 deltorphin-I 5-aminopentylamide (Fluo-DLT) in all three types of DRG neurons (small, medium, and large). In contrast, chronic inflammatory pain induced by the injection of complete Freund's adjuvant (CFA) into one hindpaw selectively increased Fluo-DLT internalization in small and medium-sized DRG neurons ipsilateral to the inflammation. Based on our previous studies in the spinal cord of mu-opioid receptor (muOR) knock-out mice, it may be assumed that the enhanced membrane recruitment of deltaORs observed after sustained morphine is attributable to stimulation of muORs. However, the selectivity of the effect induced by inflammatory pain suggests that it involves a different mechanism, namely a modality-specific and pain-related activation of C and Adelta fibers. Indeed, stimulation by capsaicin of transient receptor potential vanilloid 1 receptors, which are selectively expressed by small diameter (less than 600 microm2) DRG neurons, increased Fluo-DLT internalization exclusively in this cell population. The present results, therefore, demonstrate that DRG neurons express perikaryal deltaORs accessible to CSF-circulating ligands and that the density and, hence, presumably also the responsiveness, of these receptors may be modulated by both pain-related stimuli and sustained exposure to muOR agonists. | | 16421315
 |
Comparison of immunoblotted delta opioid receptor proteins expressed in the adult rat brain and their regulation by growth hormone.
Persson, Anders I, et al.
Neurosci. Res., 52: 1-9 (2005)
2004
| | 15811547
 |