Epstein-Barr virus-induced epigenetic alterations following transient infection.
Queen, KJ; Shi, M; Zhang, F; Cvek, U; Scott, RS
International journal of cancer. Journal international du cancer
132
2076-86
2013
Afficher le résumé
Epstein-Barr virus (EBV) is a known tumor virus associated with an increasing array of malignancies; however, the association of the virus with certain malignancies is often erratic. To determine EBV's contributions to tumorigenesis in a setting of incomplete association, a transient model of infection was established where a clonal CCL185 carcinoma cell line infected with recombinant EBV was allowed to lose viral genomes by withdrawal of selection pressure. Global gene expression comparing EBV-negative, transiently infected clones to uninfected controls identified expression changes in more than 1,000 genes. Among downregulated genes, several genes known to be deoxyribonucleic acid (DNA) methylated in cancer were identified including E-cadherin and PYCARD. A cadherin switch, increased motility and enhanced cellular invasiveness present in EBV-positive cells were retained after viral loss, indicating an epigenetic effect. Repression of PYCARD expression was a result of increased promoter CpG methylation, whereas loss of E-cadherin expression after transient EBV infection did not correlate with increased DNA methylation of the E-cadherin promoter. Rather, repression of E-cadherin was consistent with the formation of a repressive chromatin state. Decreased histone 3 or 4 acetylation at the promoter and 5' end of the E-cadherin gene was observed in an EBV-negative, transiently infected clone relative to the uninfected controls. These results suggest that EBV can stably alter gene expression in a heritable fashion in formerly infected cells, whereas its own contribution to the oncogenic process is masked. | Western Blotting | 23047626
 |
Two novel EGFP insertion alleles reveal unique aspects of Pax2 function in embryonic and adult kidneys.
Soofi, A; Levitan, I; Dressler, GR
Developmental biology
365
241-50
2011
Afficher le résumé
The Pax2 gene encodes a DNA binding protein with multiple functions in the developing intermediate mesoderm and urogenital tract. Loss of Pax2 in mice results in the complete absence of kidneys, ureters, and sex specific epithelial structures derived from the intermediate mesoderm in both males and females. In this report, we describe two new alleles of Pax2 created by inserting the enhanced green fluorescent protein coding region into the 5' untranslated leader sequence. One allele is a hypomorph that generates less protein and exhibits structural defects in kidneys and ureters upon homozygosity. A second allele is a true null that can be used to image Pax2 expressing cells in a mutant background. Organ culture and embryo analyses point to a loss of epithelial cell polarity and increased mobility in cells that have deleted Pax2 function. These experiments provide new insight into the role of Pax2 protein levels in determining correct renal architecture and cell fate. These new Pax2 alleles are valuable genetic reagents for in vivo studies of urogenital development. | Immunofluorescence | 22410172
 |
Runt-related transcription factor 3 reverses epithelial-mesenchymal transition in hepatocellular carcinoma.
Shigetomi Tanaka,Hidenori Shiraha,Yutaka Nakanishi,Shin-Ichi Nishina,Minoru Matsubara,Shigeru Horiguchi,Nobuyuki Takaoka,Masaya Iwamuro,Junro Kataoka,Kenji Kuwaki,Hiroaki Hagihara,Junichi Toshimori,Hideki Ohnishi,Akinobu Takaki,Shinichiro Nakamura,Kazuhiro Nouso,Takahito Yagi,Kazuhide Yamamoto
International journal of cancer. Journal international du cancer
131
2011
Afficher le résumé
Loss or decreased expression of runt-related transcription factor 3 (RUNX3), a tumor suppressor gene involved in gastric and other cancers, has been frequently observed in hepatocellular carcinoma (HCC). The objective of this study was to identify the regulatory mechanism of the epithelial-mesenchymal transition (EMT) by RUNX3 in HCC. Human HCC cell lines, Hep3B, Huh7, HLF and SK-Hep1, were divided into low- and high-EMT lines, based on their expression of TWIST1 and SNAI2, and were used in this in vitro study. Ectopic RUNX3 expression had an anti-EMT effect in low-EMT HCC cell lines characterized by increased E-cadherin expression and decreased N-cadherin and vimentin expression. RUNX3 expression has previously been reported to reduce jagged-1 (JAG1) expression; therefore, JAG1 ligand peptide was used to reinduce EMT in RUNX3-expressing low-EMT HCC cells. Immunohistochemical analyses were performed for RUNX3, E-cadherin, N-cadherin and TWIST1 in 33 human HCC tissues, also divided into low- and high-EMT HCC, based on TWIST1 expression. E-cadherin expression was correlated positively and N-cadherin expression was correlated negatively with RUNX3 expression in low-EMT HCC tissues. Correlations between EMT markers and RUNX3 mRNA expression were analyzed using Oncomine datasets. Similarly, mRNA expression of E-cadherin was also significantly correlated with that of RUNX3 in low-EMT HCC, while mRNA expression of JAG1 was negatively correlated with that of RUNX3. These results suggest a novel mechanism by which loss or decreased expression of RUNX3 induces EMT via induction of JAG1 expression in low-EMT HCC. | | 22488108
 |
Epithelial-mesenchymal transition biomarkers and support vector machine guided model in preoperatively predicting regional lymph node metastasis for rectal cancer.
Fan, XJ; Wan, XB; Huang, Y; Cai, HM; Fu, XH; Yang, ZL; Chen, DK; Song, SX; Wu, PH; Liu, Q; Wang, L; Wang, JP
British journal of cancer
106
1735-41
2011
Afficher le résumé
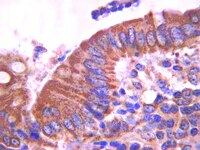
Current imaging modalities are inadequate in preoperatively predicting regional lymph node metastasis (RLNM) status in rectal cancer (RC). Here, we designed support vector machine (SVM) model to address this issue by integrating epithelial-mesenchymal-transition (EMT)-related biomarkers along with clinicopathological variables.Using tissue microarrays and immunohistochemistry, the EMT-related biomarkers expression was measured in 193 RC patients. Of which, 74 patients were assigned to the training set to select the robust variables for designing SVM model. The SVM model predictive value was validated in the testing set (119 patients).In training set, eight variables, including six EMT-related biomarkers and two clinicopathological variables, were selected to devise SVM model. In testing set, we identified 63 patients with high risk to RLNM and 56 patients with low risk. The sensitivity, specificity and overall accuracy of SVM in predicting RLNM were 68.3%, 81.1% and 72.3%, respectively. Importantly, multivariate logistic regression analysis showed that SVM model was indeed an independent predictor of RLNM status (odds ratio, 11.536; 95% confidence interval, 4.113-32.361; Pless than 0.0001).Our SVM-based model displayed moderately strong predictive power in defining the RLNM status in RC patients, providing an important approach to select RLNM high-risk subgroup for neoadjuvant chemoradiotherapy. | Immunohistochemistry | 22538975
 |
The cadherin-catenin complex as a focal point of cell adhesion and signalling: new insights from three-dimensional structures.
Gooding, Jane M, et al.
Bioessays, 26: 497-511 (2004)
2004
Afficher le résumé
Cadherins are a large family of single-pass transmembrane proteins principally involved in Ca2+-dependent homotypic cell adhesion. The cadherin molecules comprise three domains, the intracellular domain, the transmembrane domain and the extracellular domain, and form large complexes with a vast array of binding partners (including cadherin molecules of the same type in homophilic interactions and cellular protein catenins), orchestrating biologically essential extracellular and intracellular signalling processes. While current, contrasting models for classic cadherin homophilic interaction involve varying numbers of specific repeats found in the extracellular domain, the structure of the domain itself clearly remains the main determinant of cell stability and binding specificity. Through intracellular interactions, cadherin enhances its adhesive properties binding the cytoskeleton via cytoplasmic associated factors alpha- catenin, beta-catenin and p120ctn. Recent structural studies on classic cadherins and these catenin molecules have provided new insight into the essential mechanisms underlying cadherin-mediated cell interaction and catenin-mediated cellular signalling. Remarkable structural diversity has been observed in beta-catenin recognition of other cellular factors including APC, Tcf and ICAT, proteins that contribute to or compete with cadherin/catenin functioning. | | 15112230
 |
Structure-based models of cadherin-mediated cell adhesion: the evolution continues.
Koch, A W, et al.
Cell. Mol. Life Sci., 61: 1884-95 (2004)
2004
Afficher le résumé
Cadherins are glycoproteins that are responsible for homophilic, Ca2+-dependent cell-cell adhesion and play crucial roles in many cellular adhesion processes ranging from embryogenesis to the formation of neuronal circuits in the central nervous system. Many different experimental approaches have been used to unravel the molecular basis for cadherin-mediated adhesion. In particular, several high-resolution structures have provided models for cadherin-cadherin interactions that are illuminative in many respects yet contradictory in others. This review gives an overview of the structural studies of cadherins over the past decade while focusing on recent developments that reconcile some of the earlier findings. | | 15289931
 |