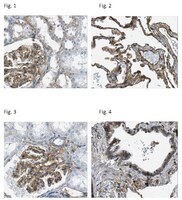
Endothelial destabilization by angiopoietin-2 via integrin β1 activation.
Hakanpaa, L; Sipila, T; Leppanen, VM; Gautam, P; Nurmi, H; Jacquemet, G; Eklund, L; Ivaska, J; Alitalo, K; Saharinen, P
Nature communications
6
5962
2015
Afficher le résumé
Angiopoietins regulate vascular homeostasis via the endothelial Tie receptor tyrosine kinases. Angiopoietin-1 (Ang1) supports endothelial stabilization via Tie2 activation. Angiopoietin-2 (Ang2) functions as a context-dependent Tie2 agonist/antagonist promoting pathological angiogenesis, vascular permeability and inflammation. Elucidating Ang2-dependent mechanisms of vascular destablization is critical for rational design of angiopoietin antagonists that have demonstrated therapeutic efficacy in cancer trials. Here, we report that Ang2, but not Ang1, activates β1-integrin, leading to endothelial destablization. Autocrine Ang2 signalling upon Tie2 silencing, or in Ang2 transgenic mice, promotes β1-integrin-positive elongated matrix adhesions and actin stress fibres, regulating vascular endothelial-cadherin-containing cell-cell junctions. The Tie2-silenced monolayer integrity is rescued by β1-integrin, phosphoinositide-3 kinase or Rho kinase inhibition, and by re-expression of a membrane-bound Tie2 ectodomain. Furthermore, Tie2 silencing increases, whereas Ang2 blocking inhibits transendothelial tumour cell migration in vitro. These results establish Ang2-mediated β1-integrin activation as a promoter of endothelial destablization, explaining the controversial vascular functions of Ang1 and Ang2. | Immunofluorescence | 25635707
 |
Compensatory redistribution of neuroligins and N-cadherin following deletion of synaptic β1-integrin.
Steven Mortillo,Alice Elste,Yongchao Ge,Shekhar B Patil,Kuangfu Hsiao,George W Huntley,Ronald L Davis,Deanna L Benson
The Journal of comparative neurology
520
2011
Afficher le résumé
β1-containing integrins are required for persistent synaptic potentiation in hippocampus and regulate hippocampal-dependent learning. Based largely on indirect evidence, there is a prevailing assumption that β1-integrins are localized at synapses, where they contribute to synapse adhesion and signaling, but this has not been examined directly. Here we investigate the fine localization of β1-integrin in adult mouse hippocampus using high-resolution immunogold labeling, with a particular emphasis on synaptic labeling patterns. We find that β1-integrins localize to synapses in CA1 and are concentrated postsynaptically. At the postsynaptic membrane, β1-integrins are found more commonly clustered near active zone centers rather than at the peripheral edges. In mice harboring a conditional deletion of β1-integrins, labeling for N-cadherin and neuroligins increases. Western blots show increased levels of N-cadherin in total lysates and neuroligins increase selectively in synaptosomes. These data suggest there is a dynamic, compensatory adjustment of synaptic adhesion. Such adjustment is specific only for certain cell adhesion molecules (CAMs), because labeling for SynCAM is unchanged. Together, our findings demonstrate unequivocally that β1-integrin is an integral synaptic adhesion protein, and suggest that adhesive function at the synapse reflects a cooperative and dynamic network of multiple CAM families. | | 22488504
 |
Distinct recycling of active and inactive β1 integrins.
Arjonen, A; Alanko, J; Veltel, S; Ivaska, J
Traffic (Copenhagen, Denmark)
13
610-25
2011
Afficher le résumé
Integrin trafficking plays an important role in cellular motility and cytokinesis. Integrins undergo constant endo/exocytic shuttling to facilitate the dynamic regulation of cell adhesion. Integrin activity toward the components of the extracellular matrix is regulated by the ability of these receptors to switch between active and inactive conformations. Several cellular signalling pathways have been described in the regulation of integrin traffic under different conditions. However, the interrelationship between integrin activity conformations and their endocytic fate have remained incompletely understood. Here, we have investigated the endocytic trafficking of active and inactive β1 integrins in cancer cells. Both conformers are endocytosed in a clathrin- and dynamin-dependent manner. The net endocytosis rate of the active β1 integrins is higher, whereas endocytosis of the inactive β1 integrin is counteracted by rapid recycling back to the plasma membrane via an ARF6- and early endosome antigen 1-positive compartment in an Rab4a- and actin-dependent manner. Owing to these distinct trafficking routes, the two receptor pools display divergent subcellular localization. At steady state, the inactive β1 integrin is mainly on the plasma membrane, whereas the active receptor is predominantly intracellular. These data provide new insights into the endocytic traffic of integrins and imply the possibility of a previously unappreciated crosstalk between pathways regulating integrin activity and traffic. | | 22222055
 |
Competitive binding of Rab21 and p120RasGAP to integrins regulates receptor traffic and migration.
Mai, A; Veltel, S; Pellinen, T; Padzik, A; Coffey, E; Marjomäki, V; Ivaska, J
The Journal of cell biology
194
291-306
2010
Afficher le résumé
Integrin trafficking from and to the plasma membrane controls many aspects of cell behavior including cell motility, invasion, and cytokinesis. Recruitment of integrin cargo to the endocytic machinery is regulated by the small GTPase Rab21, but the detailed molecular mechanisms underlying integrin cargo recruitment are yet unknown. Here we identify an important role for p120RasGAP (RASA1) in the recycling of endocytosed α/β1-integrin heterodimers to the plasma membrane. Silencing of p120RasGAP attenuated integrin recycling and augmented cell motility. Mechanistically, p120RasGAP interacted with the cytoplasmic domain of integrin α-subunits via its GAP domain and competed with Rab21 for binding to endocytosed integrins. This in turn facilitated exit of the integrin from Rab21- and EEA1-positive endosomes to drive recycling. Our results assign an unexpected role for p120RasGAP in the regulation of integrin traffic in cancer cells and reveal a new concept of competitive binding of Rab GTPases and GAP proteins to receptors as a regulatory mechanism in trafficking. | | 21768288
 |
Mammary-derived growth inhibitor (MDGI) interacts with integrin ?-subunits and suppresses integrin activity and invasion.
J Nevo,A Mai,S Tuomi,T Pellinen,O T Pentikäinen,P Heikkilä,J Lundin,H Joensuu,P Bono,J Ivaska
Oncogene
29
2009
Afficher le résumé
The majority of mortality associated with cancer is due to formation of metastases from the primary tumor. Adhesion mediated by different integrin heterodimers has an important role during cell migration and invasion. Protein interactions with the ?1-integrin cytoplasmic tail are known to influence integrin affinity for extracellular ligands, but regulating binding partners for the ?-subunit cytoplasmic tails have remained elusive. In this study, we show that mammary-derived growth inhibitor (MDGI) (also known as FABP-3 or H-FABP) binds directly to the cytoplasmic tail of integrin ?-subunits and its expression inhibits integrin activity. In breast cancer cell lines, MDGI expression correlates with suppression of the active conformation of integrins. This results in reduced integrin adhesion to type I collagen and fibronectin and inhibition of cell migration and invasion. In tissue microarray of 1331 breast cancer patients, patients with MDGI-positive tumors had more favorable 10-year distant disease-free survival compared with patients with MDGI-negative tumors. Our data indicate that MDGI is a novel interacting partner for integrin ?-subunits, and its expression modulates integrin activity and suppresses cell invasion in breast cancer patients. Retained MDGI expression is associated with favorable prognosis. | | 20802519
 |
Mammary-derived growth inhibitor alters traffic of EGFR and induces a novel form of cetuximab resistance.
Jonna Nevo,Elina Mattila,Teijo Pellinen,Daniel L Yamamoto,Henri Sara,Kristiina Iljin,Olli Kallioniemi,Petri Bono,Päivi Heikkilä,Heikki Joensuu,Anni Wärri,Johanna Ivaska
Clinical cancer research : an official journal of the American Association for Cancer Research
15
2009
Afficher le résumé
Only few predictive factors for the clinical activity of anti-epidermal growth factor receptor (EGFR) therapy are available. Mammary-derived growth inhibitor (MDGI) is a small cytosolic protein suggested to play a role in the differentiation of epithelial cells. Here, we have investigated the effect of MDGI expression on the EGFR signaling and cetuximab responsiveness of cancer cells. | | 19825952
 |
Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins.
Pellinen, T; Arjonen, A; Vuoriluoto, K; Kallio, K; Fransen, JA; Ivaska, J
The Journal of cell biology
173
767-80
2005
Afficher le résumé
Dynamic turnover of integrin cell adhesion molecules to and from the cell surface is central to cell migration. We report for the first time an association between integrins and Rab proteins, which are small GTPases involved in the traffic of endocytotic vesicles. Rab21 (and Rab5) associate with the cytoplasmic domains of alpha-integrin chains, and their expression influences the endo/exocytic traffic of integrins. This function of Rab21 is dependent on its GTP/GDP cycle and proper membrane targeting. Knock down of Rab21 impairs integrin-mediated cell adhesion and motility, whereas its overexpression stimulates cell migration and cancer cell adhesion to collagen and human bone. Finally, overexpression of Rab21 fails to induce cell adhesion via an integrin point mutant deficient in Rab21 association. These data provide mechanistic insight into how integrins are targeted to intracellular compartments and how their traffic regulates cell adhesion. | | 16754960
 |
Matrix metalloproteinase 1 interacts with neuronal integrins and stimulates dephosphorylation of Akt.
Conant, K; St Hillaire, C; Nagase, H; Visse, R; Gary, D; Haughey, N; Anderson, C; Turchan, J; Nath, A
The Journal of biological chemistry
279
8056-62
2004
Afficher le résumé
Several studies have demonstrated that matrix metalloproteinases (MMPs) are cytotoxic. The responsible mechanisms, however, are not well understood. MMPs may promote cytotoxicity through their ability to disrupt or degrade matrix proteins that support cell survival, and MMPs may also cleave substrates to generate molecules that stimulate cell death. In addition, MMPs may themselves act on cell surface receptors that affect cell survival. Among such receptors is the alpha(2)beta(1) integrin, a complex that has previously been linked to leukocyte death. In the present study we show that human neurons express alpha(2)beta(1) and that pro-MMP-1 interacts with this integrin complex. We also show that stimulation of neuronal cultures with MMP-1 is associated with a rapid reduction in the phosphorylation of Akt, a kinase that can influence caspase activity and cell survival. Moreover, MMP-1-associated dephosphorylation of Akt is inhibited by a blocking antibody to the alpha(2) integrin, but not by batimastat, an inhibitor of MMP-1 enzymatic activity. Such dephosphorylation is also stimulated by a catalytic mutant of pro-MMP-1. Additional studies show that MMP-1 causes neuronal death, which is significantly diminished by both a general caspase inhibitor and anti-alpha(2) but not by batimastat. Together, these results suggest that MMP-1 can stimulate dephosphorylation of Akt and neuronal death through a non-proteolytic mechanism that involves changes in integrin signaling. | | 14679206
 |
Integrin shedding as a mechanism of cellular adaptation during cardiac growth.
Edie C Goldsmith, Wayne Carver, Alex McFadden, Jack G Goldsmith, Robert L Price, Mark Sussman, Beverly H Lorell, Garth Cooper, Thomas K Borg, Edie C Goldsmith, Wayne Carver, Alex McFadden, Jack G Goldsmith, Robert L Price, Mark Sussman, Beverly H Lorell, Garth Cooper, Thomas K Borg
American journal of physiology. Heart and circulatory physiology
284
H2227-34
2003
Afficher le résumé
Integrin-mediated cell-extracellular matrix (ECM) interactions are essential for multiple cellular processes; however, little is known regarding integrin turnover during these events. Recent studies have demonstrated shedding of cell surface molecules and suggested this as a potential mechanism for integrin turnover. Confocal microscopy of mouse hearts under different physiological conditions demonstrated the presence of beta(1)-integrin-immunoreactive material in the interstitium. Culture media from neonatal rat cardiac myocytes and fibroblasts contained a 55-kDa fragment of beta(1)-integrin. Attachment to ECM components, response to phorbol 12-myristate 13-acetate stimulation, and matrix metalloproteinase inhibition assays demonstrated that fibroblasts responded differently to the fragment compared with myocytes. The beta(1)-integrin fragment stimulated myocyte attachment to collagen and the fragment itself bound a variety of ECM proteins. These studies indicate that as myocytes and fibroblasts change size and shape, cellular contacts with the ECM are altered, resulting in the liberation of a beta(1)-integrin fragment from the cell surface. Integrin shedding may represent a novel mechanism of rapidly modifying cell-ECM contacts during various cellular processes. | | 12573995
 |
Control of beta1 integrin function. Localization of stimulatory epitopes.
Wilkins, J A, et al.
J. Biol. Chem., 271: 3046-51 (1996)
1996
Afficher le résumé
The beta1 integrins can be expressed on the surface of cells in a latent form, which is activated by a variety of stimuli. As an approach to examining the transition to an active receptor, a panel of stimulatory antibodies to beta1 were produced and characterized. These antibodies induced adherence of the T-leukemic cell line Jurkat to collagen and fibronectin. Competitive antibody binding assays indicated the existence of at least three distinct epitope clusters A (B3B11, JB1B, 21C8), B (B44, 13B9), and C(N29) defined by the indicated antibodies. Two antibodies to the A site, JB1B and B3B11, were shown to localize to positions 671-703 and 657-670, respectively, of the beta1. This region is located in an area encompassing a predicted disulfide bond between linearly distant cysteines in beta1 (Cys415-Cys671). The homologous region of the beta3 integrin (490 690 and 602 690) has been shown to be one of the sites recognized by stimulatory antibodies to ligand-induced binding sites. The present results indicate the existence of multiple stimulatory regions and suggest considerable homology between the locations of beta1 and beta3 regulatory sites. | | 8621699
 |