Lumican expression, localization and antitumor activity in prostate cancer.
Coulson-Thomas, VJ; Coulson-Thomas, YM; Gesteira, TF; Andrade de Paula, CA; Carneiro, CR; Ortiz, V; Toma, L; Kao, WW; Nader, HB
Experimental cell research
319
967-81
2013
Afficher le résumé
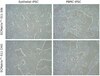
The stromal reaction surrounding tumors leads to the formation of a tumor-specific microenvironment, which may play either a restrictive role or a supportive role in the growth and progression of the tumors. Lumican, a small leucine-rich proteoglycan (SLRP) of the extracellular matrix (ECM), regulates collagen fibrillogenesis. Recently, lumican has also been shown to regulate cell behavior during embryonic development, tissue repair and tumor progression. The role of lumican in cancer varies according to the type of tumor. In this study we analyze the role of lumican in the pathogenesis of prostate cancer both in vivo and in vitro. Overall lumican up-regulation was observed in the primary tumors analyzed through both real-time PCR and immunostaining. The increase in lumican expression was observed in the reactive stroma surrounding prostate primary tumors with fibrotic deposition surrounding the acinar glands. In vitro analysis demonstrated that lumican inhibited both the migration and invasion of metastatic prostate cancer cells isolated from lymph node, bone and brain. Moreover, prostate cancer cells seeded on lumican presented a decrease in the formation of cellular projections, lamellipodia detected by a decreased rearrangement in ZO-1, keratin 8/18, integrin β1 and MT1-MMP, and invadopodia detected by disruption of α-smooth muscle actin, cortactin and N-WASP. Moreover, a significant increase in prostate cancer cell invasion was observed through the peritoneum of lumican knockout mice, further demonstrating the restrictive role lumican present in the ECM has on prostate cancer invasion. In conclusion, lumican present in the reactive stroma surrounding prostate primary tumors plays a restrictive role on cancer progression, and we therefore postulate that lumican could be a valuable marker in prostate cancer staging. | 23399832
 |
High expression of N-acetylglucosaminyltransferase IVa promotes invasion of choriocarcinoma.
Niimi, K; Yamamoto, E; Fujiwara, S; Shinjo, K; Kotani, T; Umezu, T; Kajiyama, H; Shibata, K; Ino, K; Kikkawa, F
British journal of cancer
107
1969-77
2011
Afficher le résumé
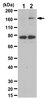
Gestational trophoblastic diseases (GTDs) are related to trophoblasts, and human chorionic gonadotropin (hCG) is secreted by GTDs as well as normal placentas. However, the asparagine-linked sugar chains on hCG contain abnormal biantennary structures in invasive mole and choriocarcinoma, but not normal pregnancy or hydatidiform mole. N-acetylglucosaminyltransferase-IV (GnT-IV) catalyses β1,4-N-acetylglucosamine branching on asparagine-linked oligosaccharides, which are consistent with the abnormal sugar chain structures on hCG.We investigated GnT-IVa expression in GTDs and placentas by immunohistochemistry, western blot, and RT-PCR. We assessed the effects of GnT-IVa knockdown in choriocarcinoma cells in vitro and in vivo.The GnT-IVa was highly expressed in trophoblasts of invasive mole and choriocarcinoma, and moderately in extravillous trophoblasts during the first trimester, but not in hydatidiform mole or other normal trophoblasts. The GnT-IVa knockdown in choriocarcinoma cells significantly reduced migration and invasive capacities, and suppressed cellular adhesion to extracellular matrix proteins. The extent of β1,4-N-acetylglucosamine branching on β1 integrin was greatly reduced by GnT-IVa knockdown, although the expression of β1 integrin was not changed. In vivo studies further demonstrated that GnT-IVa knockdown suppressed tumour engraftment and growth.These findings suggest that GnT-IVa is involved in regulating invasion of choriocarcinoma through modifications of the oligosaccharide chains of β1 integrin. | 23169300
 |
N-acetylglucosaminyltransferase V regulates extravillous trophoblast invasion through glycosylation of alpha5beta1 integrin.
Yamamoto, E; Ino, K; Miyoshi, E; Inamori, K; Abe, A; Sumigama, S; Iwase, A; Kajiyama, H; Shibata, K; Nawa, A; Kikkawa, F
Endocrinology
150
990-9
2009
Afficher le résumé
For successful human placentation, invasion of trophoblast cells into the uterus and its associated vasculature is essential, and the regulation of this process is controlled by many factors at the fetal-maternal interface. N-acetylglucosaminyltransferase V (GnT-V) is a key enzyme that catalyzes beta1, 6-N-acetylglucosamine (beta1-6GlcNAc) branching on asparagine-linked oligosaccharides of cell proteins. GnT-V and its product, beta1-6GlcNAc, are known to regulate cellular transformation and correlate with the metastatic potential of various cancer cells. The aim of the present study was to determine whether extravillous trophoblast (EVT) expressed this molecule and examine the role of GnT-V in the regulation of human trophoblast invasion. Immunohistochemistry showed that GnT-V was strongly expressed within the cytoplasm of EVT in the anchoring villi; this expression was down-regulated in EVTs invading the decidua. Suppression of beta1-6GlcNAc glycosylation by swainsonine enhanced the migratory potential and invasive capability of both primary EVTs and the EVT cell line, HTR-8/SVneo. Down-regulation of GnT-V expression by small interfering RNA in the choriocarcinoma cell line Jar consistently enhanced the migration and invasive capacity of these cells and elevated cellular adhesion to extracellular matrix proteins, such as fibronectin and collagen type I/IV. The extent of beta1-6 branching of alpha5beta1 integrin was significantly reduced in small interfering GnT-V-transfected Jar cells compared with mock transfectants, although the expression of alpha1, alpha5, alpha6, and beta1 integrin on the cell surface was not changed. These results suggest that GnT-V is expressed in human EVT and is involved in regulating trophoblast invasion through modifications of the oligosaccharide chains of alpha5beta1 integrin. | 18845630
 |
MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting alpha(2)beta(1) integrin activation.
Chen, Peter, et al.
PLoS ONE, 4: e6565 (2009)
2009
Afficher le résumé
BACKGROUND: Lung injury promotes the expression of matrix metalloproteinase-7 (MMP7, matrilysin), which is required for neutrophil recruitment and re-epithelialization. MMP7 governs the lung inflammatory response through the shedding of syndecan-1. Because inflammation and repair are related events, we evaluated the role of syndecan-1 shedding in lung re-epithelialization. METHODOLOGY/PRINCIPAL FINDING: Epithelial injury induced syndecan-1 shedding from wild-type epithelium but not from Mmp7(-/-) mice in vitro and in vivo. Moreover, cell migration and wound closure was enhanced by MMP7 shedding of syndecan-1. Additionally, we found that syndecan-1 augmented cell adhesion to collagen by controlling the affinity state of the alpha(2)beta(1) integrin. CONCLUSION/SIGNIFICANCE: MMP7 shedding of syndecan-1 facilitates wound closure by causing the alpha(2)beta(1) integrin to assume a less active conformation thereby removing restrictions to migration. MMP7 acts in the lungs to regulate inflammation and repair, and our data now show that both these functions are controlled through the shedding of syndecan-1. | 19668337
 |
Expression of N-acetylglucosaminyltransferase V in endometrial cancer correlates with poor prognosis.
Yamamoto, E; Ino, K; Miyoshi, E; Shibata, K; Takahashi, N; Kajiyama, H; Nawa, A; Nomura, S; Nagasaka, T; Kikkawa, F
British journal of cancer
97
1538-44
2007
Afficher le résumé
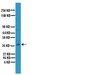
N-acetylglucosaminyltransferase V (GnT-V) is an enzyme that catalyses beta1-6 branching of N-acetylglucosamine on asparagine-linked oligosaccharides of cell proteins. The present study aimed to investigate GnT-V expression and its prognostic significance in endometrial cancer. N-acetylglucosaminyltransferase V expression was studied by immunohistochemistry in 74 surgically resected endometrial cancers, and the staining intensity was evaluated. High GnT-V expression in tumour cells was found in 43 (58.1%) of the 74 cases, and was positively correlated with advanced patient age, histological grade, and lymph vascular space involvement. Patients with high GnT-V expression had significantly impaired overall survival and progression-free survival (PFS) (P=0.0041 and P=0.0023, respectively) compared to patients with low expression of GnT-V. On multivariate analysis, GnT-V expression was an independent prognostic factor for PFS (P=0.0364). beta1-6 branching of asparagine-linked oligosaccharides was also detected in GnT-V-positive endometrial cancer cells by leukoagglutinating phytohaemagglutinin (L(4)-PHA) staining, and the molecular size of the major glycoproteins recognised by L(4)-PHA was approximately 60-200 kDa by lectin blot analysis. These results suggested that high GnT-V expression was correlated with an unfavourable clinical outcome, and that GnT-V is involved in the malignant potential of endometrial cancer by increasing the synthesis of beta1-6 branching of asparagine-linked oligosaccharides. Article en texte intégral | 17971775
 |
Caveolin-1 mediates the expression and localization of cathepsin B, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells.
Cavallo-Medved, D; Mai, J; Dosescu, J; Sameni, M; Sloane, BF
Journal of cell science
118
1493-503
2004
Afficher le résumé
Cathepsin B and pro-urokinase plasminogen activator (pro-uPA) localize to the caveolae of HCT 116 human colorectal carcinoma cells, an association mediated by active K-RAS. In this study, we established a stable HCT 116 cell line with a gene encoding antisense caveolin-1 (AS-cav-1) to examine the effects of caveolin-1, the main structural protein of caveolae, on the expression and localization of cathepsin B and pro-uPA, and their cell-surface receptors p11 and uPA receptor (uPAR), respectively. AS-cav-1 HCT 116 cells secreted less procathepsin B than control (empty vector) cells as measured by immunoblotting and pepsin activation of the proenzyme. Expression and secretion of pro-uPA was also downregulated in AS-cav-1 HCT 116 cells. Localization of cathepsin B and pro-uPA to caveolae was reduced in AS-cav-1 HCT 116 cells, and these cells expressed less total and caveolae-associated p11 and uPAR compared with control cells. Previous studies have shown that uPAR forms a complex with caveolin-1 and beta1-integrin, and we here show that downregulation of caveolin-1 also suppressed the localization of beta1-integrin to caveolae of these cells. Finally, downregulation of caveolin-1 in HCT 116 cells inhibited degradation of the extracellular matrix protein collagen IV and the invasion of these cells through Matrigel. Based on these results, we hypothesize that caveolin-1 affects the expression and localization of cathepsin B and pro-uPA, and their receptors, thereby mediating cell-surface proteolytic events associated with invasion of colon cancer cells. | 15769846
 |
Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling.
Egle Avizienyte, Anne W Wyke, Robert J Jones, Gordon W McLean, M Andrew Westhoff, Valerie G Brunton, Margaret C Frame
Nature cell biology
4
632-8
2002
Afficher le résumé
Although Src expression and activity are often elevated in colon cancer, the precise consequences of overexpression of the non-catalytic Src homology (SH) domains, or enhanced catalytic activity, are unknown. We show that, in KM12C colon cancer cells, elevated Src activity causes the components of adherens junctions, including vinculin, to be redistributed to Src-induced integrin adhesion complexes. Specifically, elevated Src activity blocks proper assembly of cell cell contacts after cells are switched from media containing a low level of calcium to media containing a high level of calcium, and E-cadherin remains internalized. In contrast, although elevated expression of the non-catalytic domains of Src is sufficient to induce assembly of integrin adhesion complexes, it does not induce disorganization of E-cadherin-associated intercellular contacts. Surprisingly, Src-induced disruption of E-cadherin localization requires specific integrin signalling, because E-cadherin redistribution is blocked by loss of cell-matrix interaction, or by inhibitory antibodies to alpha(v) or beta(1) integrin subunits. Furthermore, phosphorylation of the integrin-regulated focal adhesion kinase (FAK) on Src-specific sites is required for Src-induced de-regulation of E-cadherin, demonstrating interdependence between integrin-induced signals and cadherin-associated adhesion changes induced by Src. | 12134161
 |
The cysteine-rich domain of human ADAM 12 supports cell adhesion through syndecans and triggers signaling events that lead to beta1 integrin-dependent cell spreading.
Iba, K, et al.
J. Cell Biol., 149: 1143-56 (2000)
1999
Afficher le résumé
The ADAMs (a disintegrin and metalloprotease) family of proteins is involved in a variety of cellular interactions, including cell adhesion and ecto- domain shedding. Here we show that ADAM 12 binds to cell surface syndecans. Three forms of recombinant ADAM 12 were used in these experiments: the cys-teine-rich domain made in Escherichia coli (rADAM 12-cys), the disintegrin-like and cysteine-rich domain made in insect cells (rADAM 12-DC), and full-length human ADAM 12-S tagged with green fluorescent protein made in mammalian cells (rADAM 12-GFP). Mesenchymal cells specifically and in a dose-dependent manner attach to ADAM 12 via members of the syndecan family. After binding to syndecans, mesenchymal cells spread and form focal adhesions and actin stress fibers. Integrin beta1 was responsible for cell spreading because function-blocking monoclonal antibodies completely inhibited cell spreading, and chondroblasts lacking beta1 integrin attached but did not spread. These data suggest that mesenchymal cells use syndecans as the initial receptor for the ADAM 12 cysteine-rich domain-mediated cell adhesion, and then the beta1 integrin to induce cell spreading. Interestingly, carcinoma cells attached but did not spread on ADAM 12. However, spreading could be efficiently induced by the addition of either 1 mM Mn(2+) or the beta1 integrin-activating monoclonal antibody 12G10, suggesting that in these carcinoma cells, the ADAM 12-syndecan complex fails to modulate the function of beta1 integrin. | 10831617
 |
Regulation of integrin function: evidence that bivalent-cation-induced conformational changes lead to the unmasking of ligand-binding sites within integrin alpha5 beta1.
Mould, A P, et al.
Biochem. J., 331 ( Pt 3): 821-8 (1998)
1998
Afficher le résumé
The molecular mechanisms that regulate integrin-ligand binding are unknown; however, bivalent cations are essential for integrin activity. According to recent models of integrin tertiary structure, sites involved in ligand recognition are located on the upper face of the seven-bladed beta-propeller formed by the N-terminal repeats of the alpha subunit and on the von Willebrand factor A-domain-like region of the beta subunit. The epitopes of function-altering monoclonal antibodies (mAbs) cluster in these regions of the alpha and beta subunits; hence these mAbs can be used as probes to detect changes in the exposure or shape of the ligand-binding sites. Bivalent cations were found to alter the apparent affinity of binding of the inhibitory anti-alpha5 mAbs JBS5 and 16, the inhibitory anti-beta1 mAb 13, and the stimulatory anti-beta1 mAb 12G10 to alpha5 beta1. Analysis of the binding of these mAbs to alpha5beta1 over a range of Mn2+, Mg2+ or Ca2+ concentrations demonstrated that there was a concordance between the ability of cations to elicit conformational changes and the ligand-binding potential of alpha5 beta1. Competitive ELISA experiments provided evidence that the domains of the alpha5 and beta1 subunits recognized by mAbs JBS5/16 and 13/12G10 are spatially close, and that the distance between these two domains is increased when alpha5 beta1 is occupied by bivalent cations. Taken together, our findings suggest that bivalent cations induce a conformational relaxation in the integrin that results in exposure of ligand-binding sites, and that these sites lie near an interface between the alpha subunit beta-propeller and the beta subunit putative A-domain. | 9560310
 |
Identification of a novel anti-integrin monoclonal antibody that recognises a ligand-induced binding site epitope on the beta 1 subunit.
Mould, A P, et al.
FEBS Lett., 363: 118-22 (1995)
1994
Afficher le résumé
Integrins are the major family of receptors involved in the adhesive interactions of cells with extracellular matrix macromolecules. Although it is known that integrins can exist in active or inactive states, the molecular mechanisms by which integrin activity is modulated are poorly understood. A novel anti-integrin monoclonal antibody, 12G10, that enhances alpha 5 beta 1-fibronectin interactions has been identified. 12G10 binds to the beta 1 subunit and appears to recognise a region of the subunit that contains the epitopes of several previously described activating or inhibitory monoclonal antibodies. However, unlike other activating anti-beta 1 antibodies, the binding of 12G10 to alpha 5 beta 1 is increased in the presence of ligands (fibronectin fragment or RGD peptide). This is the first report for the beta 1 integrin family of an antibody that recognises a ligand-induced binding site, and further emphasises the functional importance of a specific region of the beta 1 subunit in regulating integrin-ligand interactions. | 7537221
 |