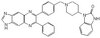
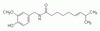
Histone deacetylase inhibitors enhance the chemosensitivity of tumor cells with cross-resistance to a wide range of DNA-damaging drugs.
Kei-ichi Ozaki, Futaba Kishikawa, Masashi Tanaka, Toshiaki Sakamoto, Susumu Tanimura, Michiaki Kohno
Cancer science
99
376-84
2008
Afficher le résumé
Although DNA-damaging agents are among the most effective anticancer drugs in clinical use, their overall effectiveness is limited by the development of cross-resistance to these drugs. Given that histone deacetylase (HDAC) inhibitors increase the acetylation of core histones, resulting in an open chromatin configuration that is more accessible to DNA-targeting agents, we examined whether HDAC inhibitors might enhance the cytotoxicity of DNA-damaging drugs in six human ovarian tumor cell lines that exhibit different cisplatin sensitivities. Low concentrations of HDAC inhibitors, which alone exhibited little cytotoxicity, markedly enhanced the induction of apoptotic cell death not only by cisplatin but also by a wide variety of DNA-targeting anticancer drugs in these tumor cell lines, irrespective of their sensitivities to the respective drugs. In contrast, HDAC inhibitors did not increase the cytotoxicity of metabolic antagonists or microtubule-targeting agents. HDAC inhibitors potentiated both the phosphorylation of histone H2AX on serine-139 (a marker of DNA double-strand breaks) as well as the accumulation of reactive oxygen species induced by DNA-damaging agents in tumor cells. The enhanced generation of reactive oxygen species appeared to be responsible for the enhanced apoptotic cell death induced by the combination of these drugs. These results indicate that the combination of an HDAC inhibitor with a wide variety of DNA-damaging agents is a promising chemotherapeutic strategy for the eradication of tumor cells, regardless of whether the cells are sensitive or resistant to the DNA-damaging anticancer drugs. | 18201278
 |
Kaposi's sarcoma tumor cells preferentially express Bcl-xL.
Foreman, K E, et al.
Am. J. Pathol., 149: 795-803 (1996)
1996
Afficher le résumé
Several recently identified proteins such as Bcl-2 and Bcl-x have been found to regulate programmed cell death (i.e., apoptosis). In this report, we examined the levels of expression of proteins that can either prevent apoptosis (i.e., Bcl-2 or the long form of Bcl-x, designated Bcl-x1) or promote apoptosis (i.e., Bax or the short form of Bcl-x, designated Bcl-xs) in proliferating benign and malignant endothelial cells (ECs). In normal skin with quiescent ECs, no detection by immunohistochemical staining was observed for Bcl-xL, Bcl-xs, or Bcl-2. However, in diseased skin samples that feature a prominent angiogenic response such as in psoriasis or pyogenic granulomas, the proliferating ECs markedly overexpressed Bcl-xL, with little to no Bcl-2. In an acquired-immune-deficiency-syndrome-related neoplasm, Kaposi's sarcoma, the spindle-shaped tumor cells also overexpressed Bcl-xL compared with Bcl-2. These in vivo studies were extended in vitro using cultured ECs and Kaposi's sarcoma tumor cells that were examined by flow cytometry and immunoblot analysis. Both cultured ECs and Kaposi's sarcoma tumor cells express significantly higher levels of Bcl-xL than Bcl-2. Such overexpression of cell survival gene products may contribute to prolonging the longevity of EC-derived cells in several different benign and neoplastic skin disorders that are characterized by a prominent angiogenic tissue response. | 8780384
 |
Regulation of lymphocyte survival by the bcl-2 gene family.
Cory, S
Annu. Rev. Immunol., 13: 513-43 (1995)
1994
Afficher le résumé
The control of cell survival is of central importance in tissues with high cell turnover such as the lymphoid system, and its disruption may be a critical step in tumorigenesis. Genes homologous to bcl-2, the oncogene implicated in human follicular lymphoma, play a key role in regulating physiologic cell death (apoptosis). Bcl-2 and its relatives bcl-x and bax encode intracellular membrane-bound proteins that share homology in three domains with a wider family of viral and cellular proteins. The Bcl-2 and Bcl-x proteins enhance the survival of lymphocytes and other cell types but do not promote their proliferation. High levels of Bax or of a smaller Bcl-x variant antagonize the survival function of Bcl-2. The mechanism by which Bcl-2 promotes cell survival remains unknown, but it appears to require association with Bax. Bcl-2 may combat the action of cysteine proteases thought to trigger apoptosis. Bcl-2 is not essential for embryogenesis or lymphoid development. However, upregulation of Bcl-2 appears to be the normal mechanism for positive selection of developing lymphocytes, and its continued expression is critical for survival of mature peripheral B and T cells. Constitutive expression of Bcl-2 does not abrogate deletion of self-reactive lymphocytes, nor disturb T lymphoid homeostasis; however, it substantially increases the pool of mature noncycling B cells. The risk of B lymphoid tumors is also enhanced, probably because Bcl-2 can countermand the apoptotic action of other oncoproteins such as Myc. Expression in tumors of bcl-2 and other cell survival genes may constitute a major barrier to the success of genotoxic cancer therapy. | 7612233
 |