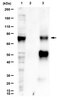
The glycoprotein of vesicular stomatitis virus promotes release of virus-like particles from tetherin-positive cells.
Brinkmann, C; Hoffmann, M; Lübke, A; Nehlmeier, I; Krämer-Kühl, A; Winkler, M; Pöhlmann, S
PLoS One
12
e0189073
2017
Show Abstract
Vesicular stomatitis virus (VSV) release from infected cells is inhibited by the interferon (IFN)-inducible antiviral host cell factor tetherin (BST-2, CD317). However, several viruses encode tetherin antagonists and it is at present unknown whether residual VSV spread in tetherin-positive cells is also promoted by a virus-encoded tetherin antagonist. Here, we show that the viral glycoprotein (VSV-G) antagonizes tetherin in transfected cells, although with reduced efficiency as compared to the HIV-1 Vpu protein. Tetherin antagonism did not involve alteration of tetherin expression and was partially dependent on a GXXXG motif in the transmembrane domain of VSV-G. However, mutation of the GXXXG motif did not modulate tetherin sensitivity of infectious VSV. These results identify VSV-G as a tetherin antagonist in transfected cells but fail to provide evidence for a contribution of tetherin antagonism to viral spread. | 29216247
 |
Characterization of Vesicular Stomatitis Virus Pseudotypes Bearing Essential Entry Glycoproteins gB, gD, gH, and gL of Herpes Simplex Virus 1.
Rogalin, HB; Heldwein, EE
J Virol
90
10321-10328
2016
Show Abstract
Herpes simplex viruses (HSVs) are unusual in that unlike most enveloped viruses, they require at least four entry glycoproteins, gB, gD, gH, and gL, for entry into target cells in addition to a cellular receptor for gD. The dissection of the herpes simplex virus 1 (HSV-1) entry mechanism is complicated by the presence of more than a dozen proteins on the viral envelope. To investigate HSV-1 entry requirements in a simplified system, we generated vesicular stomatitis virus (VSV) virions pseudotyped with HSV-1 essential entry glycoproteins gB, gD, gH, and gL but lacking the native VSV fusogen G. These virions, referred to here as VSVΔG-BHLD virions, infected a cell line expressing a gD receptor, demonstrating for the first time that the four essential entry glycoproteins of HSV-1 are not only required but also sufficient for cell entry. To our knowledge, this is the first time the VSV pseudotyping system has been successfully extended beyond two proteins. Entry of pseudotyped virions required a gD receptor and was inhibited by HSV-1 specific anti-gB or anti-gH/gL neutralizing antibodies, which suggests that membrane fusion during the entry of the pseudotyped virions shares common requirements with the membrane fusion involved in HSV-1 entry and HSV-1-mediated syncytium formation. The HSV pseudotyping system established in this study presents a novel tool for systematic exploration of the HSV entry and membrane fusion mechanisms.Herpes simplex viruses (HSVs) are human pathogens that can cause cold sores, genital herpes, and blindness. No vaccines or preventatives are available. HSV entry into cells-a prerequisite for a successful infection-is a complex process that involves multiple viral and host proteins and occurs by different routes. Detailed mechanistic knowledge of the HSV entry is important for understanding its pathogenesis and would benefit antiviral and vaccine development, yet the presence of more than a dozen proteins on the viral envelope complicates the dissection of the HSV entry mechanisms. In this study, we generated heterologous virions displaying the four essential entry proteins of HSV-1 and showed that they are capable of cell entry and, like HSV-1, require all four entry glycoproteins along with a gD receptor. This HSV pseudotyping system pioneered in this work opens doors for future systematic exploration of the herpesvirus entry mechanisms. | 27605677
 |
Impact of Vesicular Stomatitis Virus M Proteins on Different Cellular Functions.
Redondo, N; Madan, V; Alvarez, E; Carrasco, L
PLoS One
10
e0131137
2015
Show Abstract
Three different matrix (M) proteins termed M1, M2 and M3 have been described in cells infected with vesicular stomatitis virus (VSV). Individual expression of VSV M proteins induces an evident cytopathic effect including cell rounding and detachment, in addition to a partial inhibition of cellular protein synthesis, likely mediated by an indirect mechanism. Analogous to viroporins, M1 promotes the budding of new virus particles; however, this process does not produce an increase in plasma membrane permeability. In contrast to M1, M2 and M3 neither interact with the cellular membrane nor promote the budding of double membrane vesicles at the cell surface. Nonetheless, all three species of M protein interfere with the transport of cellular mRNAs from the nucleus to the cytoplasm and also modulate the redistribution of the splicing factor. The present findings indicate that all three VSV M proteins share some activities that interfere with host cell functions. | 26091335
 |
Generation of VSV pseudotypes using recombinant ΔG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines.
Whitt, MA
J Virol Methods
169
365-74
2010
Show Abstract
Vesicular stomatitis virus (VSV) is a prototypic enveloped animal virus that has been used extensively to study virus entry, replication and assembly due to its broad host range and robust replication properties in a wide variety of mammalian and insect cells. Studies on VSV assembly led to the creation of a recombinant VSV in which the glycoprotein (G) gene was deleted. This recombinant (rVSV-ΔG) has been used to produce VSV pseudotypes containing the envelope glycoproteins of heterologous viruses, including viruses that require high-level biocontainment; however, because the infectivity of rVSV-ΔG pseudotypes is restricted to a single round of replication the analysis can be performed using biosafety level 2 (BSL-2) containment. As such, rVSV-ΔG pseudotypes have facilitated the analysis of virus entry for numerous viral pathogens without the need for specialized containment facilities. The pseudotypes also provide a robust platform to screen libraries for entry inhibitors and to evaluate the neutralizing antibody responses following vaccination. This manuscript describes methods to produce and titer rVSV-ΔG pseudotypes. Procedures to generate rVSV-ΔG stocks and to quantify virus infectivity are also described. These protocols should allow any laboratory knowledgeable in general virological and cell culture techniques to produce successfully replication-restricted rVSV-ΔG pseudotypes for subsequent analysis. | 20709108
 |