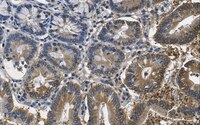
MABS1253 Sigma-AldrichAnti-O-GlcNAc Antibody, clone RL1
Detect GlcNAcylated proteins using this mouse monoclonal Anti-O-GlcNAc, clone RL1 , Cat. No. MABS1253, validated for use in Affinity Binding, Electron Microscopy, Immunocytochemistry, Immunofluorescence, Immunohistochemistry, Immunoprecipitation, Inhibition, and Western Blotting.
More>> Detect GlcNAcylated proteins using this mouse monoclonal Anti-O-GlcNAc, clone RL1 , Cat. No. MABS1253, validated for use in Affinity Binding, Electron Microscopy, Immunocytochemistry, Immunofluorescence, Immunohistochemistry, Immunoprecipitation, Inhibition, and Western Blotting. Less<<Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Species Reactivity | Key Applications | Host | Format | Antibody Type |
|---|---|---|---|---|
| R, Xn, H, Dr | ABA, EM, ICC, IF, IHC, IP, Inhibition, WB | M | Purified | Monoclonal Antibody |
| References |
|---|
| Product Information | |
|---|---|
| Format | Purified |
| Presentation | Purified mouse IgM in buffer containing PBS without preservatives. |
| Quality Level | MQ100 |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Packaging Information | |
|---|---|
| Material Size | 100 µL |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| MABS1253 | 04054839055577 |
Documentation
Anti-O-GlcNAc Antibody, clone RL1 SDS
| Title |
|---|
Anti-O-GlcNAc Antibody, clone RL1 Certificates of Analysis
| Title | Lot Number |
|---|---|
| Anti-O-GlcNAc, clone RL1 - 3524307 | 3524307 |
| Anti-O-GlcNAc, clone RL1 -Q2737199 | Q2737199 |