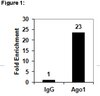
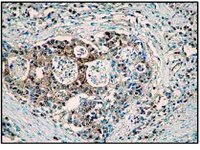
Caspase-12 mediates carbon tetrachloride-induced hepatocyte apoptosis in mice.
Liu, H; Wang, Z; Nowicki, MJ
World journal of gastroenterology
20
18189-98
2014
Show Abstract
To investigate the role of caspase-12 and its downstream targets in carbon tetrachloride (CCl4)-induced hepatocyte apoptosis.The role of caspase-12 was determined by using caspase-12 knock-out ((-/-)) mice. CCl4 (300 μL/kg body weight) or vehicle (corn oil) was administered to caspase-12(+/+) or caspase-12(-/-) mice as a single intraperitoneal injection. The animals were sacrificed 24 h after the CCl4 treatment. Blood was collected to evaluate liver function by the measurement of the activity of alanine aminotransferase. Liver samples were used for the measurements of reactive oxygen species using plasma malondialdehyde as biomarker, hepatocyte apoptosis was evaluated via terminal transferase-mediated dUTP nick-end labeling and controlled by morphologic study, and cytochrome C release and caspase activations were measured by Western blotting.Administration of a low dose of CCl4 resulted in hepatocyte apoptosis and acute liver injury in wild-type mice. CCl4 also induced the generation of reactive oxygen species and induction of endoplasmic reticulum stress in the liver followed by activations of caspase-12, -9 and -3 as well as release of small amounts of cytochrome C. However, in the CCl4-treated caspase-12(-/-) mice, activation of caspase-9 and -3 were significantly attenuated (P less than 0.05); no effect was seen in cytochrome C release. CCl4-induced apoptosis and liver damage was markedly reduced in caspase-12(-/-) mice compared to caspase-12(+/+) mice (P less than 0.05). The active form of caspase-8 was not detected in either caspase-12(+/+) or caspase-12(-/-) mice. There was no significant different in the formation of reactive oxygen species in the livers of caspase-12(+/+) and caspase-12(-/-) mice treated with CCl4.Caspase-12 plays a pivotal role in CCl4-induced hepatic apoptosis through the activation of the downstream effector caspase-3 directly and/or indirectly via caspase-9 activation. | Western Blotting | 25561786
 |
Activation of endoplasmic reticulum stress in degenerating photoreceptors of the rd1 mouse.
Yang, LP; Wu, LM; Guo, XJ; Tso, MO
Investigative ophthalmology & visual science
48
5191-8
2007
Show Abstract
Endoplasmic reticulum (ER) stress has been implicated in a wide variety of neurodegenerative disorders of the central nervous system (CNS). This study was designed to elucidate the role of ER stress in photoreceptor apoptosis in the rd1 mouse.Photoreceptor apoptosis in the rd1 mouse was detected by terminal dUTP transferase nick-end labeling (TUNEL). Protein expressions of ER stress sensors, including glucose-regulated protein-78 (GRP78/BiP), caspase-12, phospho-eukaryotic initiation factor 2alpha (eIF2alpha), and phospho-pancreatic ER kinase (PERK), were examined by immunofluorescence and Western blot assays.Accompanying photoreceptor apoptosis in the rd1 mouse, the protein expressions of GRP78/BiP, caspase-12, phospho-eIF2alpha, and phospho-PERK were upregulated in a time-dependent manner. The upregulation of these proteins coincided with or preceded photoreceptor apoptosis. At the peak of their expression, these proteins were primarily located in the photoreceptor inner segments, the outer nuclear layer, or both.ER stress plays an important role in photoreceptor apoptosis in the rd1 mouse. Therefore, ER stress modulators may be strong candidates as therapeutic agents in the treatment of retinal degenerative diseases. | | 17962473
 |
Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis.
Liu, H; Baliga, R
Journal of the American Society of Nephrology : JASN
16
1985-92
2005
Show Abstract
Reactive oxygen metabolites are important mediators in cisplatin-induced apoptosis in renal tubular epithelial cells (LLC-PK1). Mitochondria have been implicated to play a principal role in cisplatin-induced apoptosis. Caspase 12, an endoplasmic reticulum (ER)-specific caspase, participates in apoptosis under ER stress. Cytochrome P450 system is crucial to the generation of reactive oxygen metabolites and is present at high concentration in the ER. The direct role of caspase 12 in any model of renal injury has not previously been described. In this study, cleavage of procaspase 12 preceded that of caspases 3 and 9 after cisplatin treatment of LLC-PK1 cells. The active form of caspase 8 was not detected throughout the course of study. Preincubation of the LLC-PK1 cells with the caspase 9 inhibitor did not attenuate caspase 3 activation and provided no significant protection. Caspase 3 inhibitor provided only modest protection against cisplatin-induced apoptosis. LLC-PK1 cells that were transfected with anti-caspase 12 antibody significantly attenuated cisplatin-induced apoptosis. Taken together, these data indicate that caspase 12 plays a pivotal role in cisplatin-induced apoptosis. It is proposed that the oxidative stress that results from the interaction of cisplatin with the ER cytochrome P450 leads to activation of procaspase 12, resulting in apoptosis. | | 15901768
 |
Global changes in gene regulation demonstrate that unconjugated bilirubin is able to upregulate and activate select components of the endoplasmic reticulum stress response pathway.
Garth H Oakes,John R Bend
Journal of biochemical and molecular toxicology
24
2001
Show Abstract
Elevated concentrations of unconjugated bilirubin (UCB) are responsible for neonatal jaundice and can eventually lead to kernicterus or death. The molecular mechanism of UCB toxicity is incompletely elucidated. The purpose of this study was to analyze changes in gene regulation mediated by UCB to determine novel pathways that contribute to UCB-mediated toxicity. We employed microarray analysis to determine changes in gene regulation mediated by UCB at both pro- (50 microM) and antioxidant (70 nM) concentrations in Hepa 1c1c7 cells at 1 and 6 h. The changes observed in select genes were validated with qPCR. Using immunoblot analysis, we validated these changes at the protein level for select genes and documented the activation of two proteins involved in the endoplasmic reticulum (ER) stress pathway, eIF2 alpha and PERK. Following treatment with 50 microM UCB, microarray analysis revealed the upregulation of many genes involved in ER stress (ATF3, BiP, CHOP, Dnajb1, and Herp). We demonstrate that upregulation of the proapoptotic transcription factor CHOP results in increased intracellular protein content. It was determined that activation of proteins involved in ER stress was an early event in UCB toxicity as eIF2 alpha and PERK were both phosphorylated and activated by 1 h posttreatment. We also demonstrate that procaspase-12 content, a proposed initiator caspase in ER stress-mediated apoptosis, is decreased by 4 h posttreatment. In conclusion, this study demonstrates that elevated concentrations of UCB (50 microM) are able to activate select components of the ER stress pathway in Hepa 1c1c7 cells, which may contribute to UCB-mediated apoptosis. | | 20196124
 |
Characterization of seven murine caspase family members.
Van de Craen, M, et al.
FEBS Lett., 403: 61-9 (1997)
1997
Show Abstract
Seven members of the murine caspase (mCASP) family were cloned and functionally characterized by transient overexpression: mCASP-1 (mICE), mCASP-2 (Ich1), mCASP-3 (CPP32), mCASP-6 (Mch2), mCASP-7 (Mch3), mCASP-11 (TX) and mCASP-12. mCASP-11 is presumably the murine homolog of human CASP-4. Although mCASP-12 is related to human CASP-5 (ICErel-III), it is most probably a new CASP-1 family member. On the basis of sequence homology, the caspases can be divided into three subfamilies: first, mCASP-1, mCASP-11 and mCASP-12; second, mCASP-2; third, mCASP-3, mCASP-6 and mCASP-7. The tissue distribution of the CASP-1 subfamily transcripts is more restricted than that of the CASP-3 subfamily transcripts, suggesting that the transcriptional regulation of the CASP members within one subfamily is related, but is quite different between the CASP-1 and the CASP-3 subfamilies. Transient overexpression of each of the seven CASPs induced apoptosis in mammalian cells. Only two, mCASP-1 as well as mCASP-3, were able to process precursor interleukin (IL)-1beta to biologically active IL-1beta. In addition, mCASP-3 is the predominant PARP-cleaving enzyme in vivo. | | 9038361
 |
Production of matrix metalloproteinases and tissue inhibitor of metalloproteinases-1 by human brain tumors.
Nakagawa, T, et al.
J. Neurosurg., 81: 69-77 (1994)
1994
Show Abstract
The role of matrix metalloproteinases (MMP's) and their inhibitor, tissue inhibitor of metalloproteinases-1 (TIMP-1), in human brain tumor invasion was investigated. Gelatinolytic activity was assayed via gelatin zymography, and four MMP's (MMP-1, MMP-2, MMP-3, and MMP-9) and TIMP-1 were immunolocalized in human brain tumors and in normal brain tissues using monoclonal antibodies. The tissue was surgically removed from 44 patients: glioblastoma (five cases), anaplastic astrocytoma (six cases), astrocytoma (four cases), metastatic tumor (six cases), neurinoma (10 cases), meningioma (10 cases), and normal brain tissue (three cases). Glioblastomas, anaplastic astrocytomas, and metastatic tumors showed high gelatinolytic activity and positive immunostaining for MMP's; TIMP-1 was also expressed in these tumors, but some tumor cells were negative for the antibody. Astrocytomas had low gelatinolytic activity and the tumor cells showed no immunoreactivity for MMP's and TIMP-1. Although neurinomas and meningiomas had only moderate proteinase activity and exhibited positive immunoreactivity for MMP-9, intense expression of TIMP-1 was simultaneously observed in these tumor cells. These findings suggest that MMP's play an important role in human brain tumor invasion, probably due to an imbalance between the production of MMP's and TIMP-1 by the tumor cells. | | 8207529
 |