03-0175-00 MilliporeSMC™ Immunogenicity Bead Based Assay Development Kit
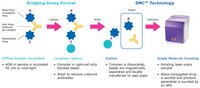
SMC™ technology enables ADA assay development by labelling the drug with capture & detection reagents and utilizing buffer reagents for optimization. It allows you to develop a homogenous species-independent assay format that is simple, easy to design and validate.
More>> SMC™ technology enables ADA assay development by labelling the drug with capture & detection reagents and utilizing buffer reagents for optimization. It allows you to develop a homogenous species-independent assay format that is simple, easy to design and validate. Less<<Productos recomendados
Descripción
| Replacement Information |
|---|
| References |
|---|
| Biological Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Storage and Shipping Information |
|---|
| Packaging Information | |
|---|---|
| Material Size | 1 Kit |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 03-0175-00 | 04054839753541 |
Documentation
Protocols
| Title |
|---|
| Protocol- 03-0175-00 |
| SMC Immunogenicity Bead Based Assay Development Kit |
SMC™ Immunogenicity Bead Based Assay Development Kit Ficha datos de seguridad (MSDS)
| Título |
|---|