SCM121 Sigma-AldrichOsteoMAX-XF™ Differentiation Medium
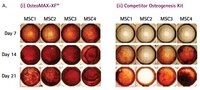
Defined Xeno-Free osteogenesis differentiation medium that is specially formulated to readily differentiate human mesenchymal stem cells (MSCs) into mature osteocytes as assessed by alizarin red & alkaline phosphatase staining.
More>> Defined Xeno-Free osteogenesis differentiation medium that is specially formulated to readily differentiate human mesenchymal stem cells (MSCs) into mature osteocytes as assessed by alizarin red & alkaline phosphatase staining. Less<<Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| Key Applications |
|---|
| CULT, Cult |
| References |
|---|
| Product Information | |
|---|---|
| Components |
|
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Cross Reactivity | None |
| Media Form | Liquid |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Packaging Information | |
|---|---|
| Material Size | 100ml/kit |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| SCM121 | 04053252847899 |
Documentation
OsteoMAX-XF™ Differentiation Medium Ficha datos de seguridad (MSDS)
| Título |
|---|
Licencias necesarias e Información técnica
| Cargo |
|---|
| A Novel Serum-free and Xeno-free Differentiation Medium for Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells |
Póster
| Cargo |
|---|
| A xeno-free, serum-free defined medium to rapidly differentiate human mesenchymal stem cells to osteoblasts |
Manuales del usuario
| Cargo |
|---|
| OsteoMAX-XFTM Differentiation Medium |