438185 Sigma-AldrichLovastatin - CAS 75330-75-5 - Calbiochem
Lovastatin, CAS 75330-75-5, is an anti-hypercholesterolemic agent that inhibits the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase.
More>> Lovastatin, CAS 75330-75-5, is an anti-hypercholesterolemic agent that inhibits the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. Less<<Sinónimos: Mevinolin, MK-803, L-Type Calcium Channel Blocker IV
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| CAS # | Empirical Formula |
|---|---|
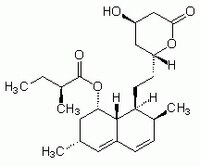
| 75330-75-5 | C₂₄H₃₆O₅ |
Products
| Número de referencia | Embalaje | Cant./Env. | |
|---|---|---|---|
| 438185-25MG | Ampolla de plást. | 25 mg |
| Product Information | |
|---|---|
| CAS number | 75330-75-5 |
| ATP Competitive | N |
| Form | White to off-white powder |
| Hill Formula | C₂₄H₃₆O₅ |
| Chemical formula | C₂₄H₃₆O₅ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ200 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | EK7907000 |
| Safety Information | |
|---|---|
| R Phrase | R: 63 Possible risk of harm to the unborn child. |
| S Phrase | S: 22-24/25 Do not breathe dust. Avoid contact with skin and eyes. |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 438185-25MG | 04055977186956 |
Documentation
Lovastatin - CAS 75330-75-5 - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
Lovastatin - CAS 75330-75-5 - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 438185 |
Referencias bibliográficas
| Visión general referencias |
|---|
| Rao, S., et al. 1999. Proc. Natl. Acad. Sci. USA 96, 7197. Carel, K., et al. 1996. J. Biol. Chem. 271, 30625. McGuire, T.F., et al. 1996. J. Biol. Chem. 271, 27402. Umetani, N., et al. 1996. Biochim. Biophys. Acta 1303, 199. Xu, X.Q., et al. 1996. Arch. Biochem. Biophys. 326, 233. Reusch, J.E.-B., et al. 1995. J. Biol. Chem. 270, 2036. Jakobisiak, M., et al. 1991. Proc. Natl. Acad. Sci. USA 88, 3628. Keyomarsi, K., et al. 1991. Cancer Res. 51, 3602. Mendola, C.E., and Backer, J.M. 1990. Cell Growth Differ. 1, 499. |