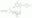370677 Sigma-AldrichGuanosine 3ʹ,5ʹ-cyclic Monophosphorothioate, 8-(4-Chlorophenylthio)-, Rp-Isomer, Triethylammonium Salt - Calbiochem
A potent, cell-permeable, and metabolically-stable inhibitor of protein kinase G Iα, Iβ, and type II.
More>> A potent, cell-permeable, and metabolically-stable inhibitor of protein kinase G Iα, Iβ, and type II. Less<<Sinónimos: Rp-8-pCPT-cGMPS, TEA, PKG Inhibitor III
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| Empirical Formula |
|---|
| C₁₆H₁₅ClN₅O₆PS₂ · C₆H₁₅N |
Products
| Número de referencia | Embalaje | Cant./Env. | |
|---|---|---|---|
| 370677-1UMOL | Ampolla de plást. | 1 umol |
| Description | |
|---|---|
| Overview | A potent, cell-permeable, and metabolically-stable inhibitor of protein kinase G Iα, Iβ, and type II. Significantly more lipophilic and membrane-permeable than Rp-cGMPS or Rp-8-Br-cGMPS (Cat. No. 370674). Note: 1 µmol = 0.61 mg. |
| Catalogue Number | 370677 |
| Brand Family | Calbiochem® |
| Synonyms | Rp-8-pCPT-cGMPS, TEA, PKG Inhibitor III |
| Product Information | |
|---|---|
| ATP Competitive | N |
| Declaration | Sold under license of U.S. Patent 5,625,056, Patents DE 3,802,865.4, and DE 4,217,679 issued to BIOLOG LSI. |
| Form | Lyophilized |
| Hill Formula | C₁₆H₁₅ClN₅O₆PS₂ · C₆H₁₅N |
| Chemical formula | C₁₆H₁₅ClN₅O₆PS₂ · C₆H₁₅N |
| Hygroscopic | Hygroscopic |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | PKG1α |
| Purity | ≥99% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 370677-1UMOL | 04055977191707 |
Documentation
Guanosine 3ʹ,5ʹ-cyclic Monophosphorothioate, 8-(4-Chlorophenylthio)-, Rp-Isomer, Triethylammonium Salt - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
Guanosine 3ʹ,5ʹ-cyclic Monophosphorothioate, 8-(4-Chlorophenylthio)-, Rp-Isomer, Triethylammonium Salt - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 370677 |
Referencias bibliográficas
| Visión general referencias |
|---|
| Abd Alla, S., et al. 1996. Eur. J. Biochem. 241, 498. Lee, S.J., et al. 1996. Dev. Biol. 180, 324. Van Uffelen, B.E., et al. 1996. J. Leukoc. Biol. 60, 94. Willmott, N., et al. 1996. J. Biol. Chem. 271, 3699. Butt, E., et al. 1994. Eur. J. Pharmacol. 269, 265. |