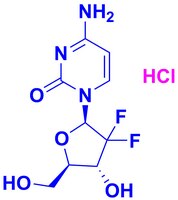
504594 Sigma-AldrichGemcitabine, HCl - CAS 122111-03-9 - Calbiochem
Sinónimos: LY188011, dFdC, dFdCyd, 2ʹ,2ʹ-Difluoro-2ʹ-deoxycytidine, HCl, 2ʹ-Deoxy-2ʹ,2ʹ-difluorocytidine, HCl, 4-Amino-1-((2R,5S)-3,3-difluoro-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one, HCl, Ribonucleotide Reductase Inhibitor II, RNR Inhibitor II
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| CAS # | Empirical Formula |
|---|---|
| 122111-03-9 | C₉H₁₁F₂N₃O₄ • HCl |
Products
| Número de referencia | Embalaje | Cant./Env. | |
|---|---|---|---|
| 5045940001 | Frasco de vidrio | 50 mg |
| Product Information | |
|---|---|
| CAS number | 122111-03-9 |
| Form | White powder |
| Hill Formula | C₉H₁₁F₂N₃O₄ • HCl |
| Chemical formula | C₉H₁₁F₂N₃O₄ • HCl |
| Hygroscopic | Hygroscopic |
| Reversible | N |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | DNA |
| Secondary target | DNA polymerase |
| Purity | ≥99% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 5045940001 | 04055977243895 |
Documentation
Gemcitabine, HCl - CAS 122111-03-9 - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
Gemcitabine, HCl - CAS 122111-03-9 - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 504594 |
Referencias bibliográficas
| Visión general referencias |
|---|
| Hung, S.W., et al. 2012. Cancer Lett. 320, 138. Fowler, J.D., et al. 2008. J. Biol. Chem. 283, 15339. Mackey, J.R., et al. 1998. Cancer Res. 58, 4349. Eda, H., et al. 1998. Cancer Res. 58, 1165. Burris III, H.A., et al. 1997. J. Clin. Oncol. 15, 2403. Heinemann, V., et al. 1992. Cancer Res. 52, 533. Hertel, L.W., et al. 1990. Cancer Res. 50, 4417. |