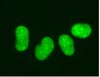
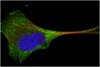
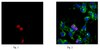
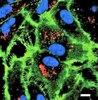
Subcellular localization and analysis of apparent 180-kDa and 220-kDa proteins of the breast cancer susceptibility gene, BRCA1
Thomas, J. E., et al
J Biol Chem, 271:28630-5 (1996)
1996
| Immunoblotting (Western), Immunoprecipitation | 8910495
 |
Ligand stimulation of a Ret chimeric receptor carrying the activating mutation responsible for the multiple endocrine neoplasia type 2B.
Rizzo, C, et al.
J. Biol. Chem., 271: 29497-501 (1996)
1996
Mostrar resumen
Inherited activating mutations of Ret, a receptor tyrosine kinase, predispose to multiple endocrine neoplasia (MEN) types 2A and 2B and familial medullary thyroid carcinoma. To investigate the effects induced by acute stimulation of Ret, we transfected both PC12 and NIH 3T3 cells with a molecular construct in which the ligand-binding domain of the epidermal growth factor receptor was fused to the catalytic domain of Ret. Acute stimulation of the chimeric receptor induced PC12 cells to express a neuronal-like phenotype. Moreover, we introduced the dominant mutation, responsible for the multiple endocrine neoplasia type 2B, in the catalytic domain of the Ret chimera. Expression of the mutant chimera, in the absence of ligand stimulation, induces the PC12 cells to acquire a flat morphology with short neuritic processes and transforms the NIH 3T3 cells. Stimulation of the mutant chimera with epidermal growth factor causes a drastic overgrowth of long neuritic processes, with the induction of the suc1-associated protein tyrosine phosphorylation in PC12 cells and higher transforming efficiency in NIH 3T3 cells. These data indicate that the gain-of-function MEN2B mutation does not abrogate ligand responsiveness of Ret and suggest that the presence of Ret ligand could play a role in the pathogenesis of the MEN2B syndrome. | Cell Culture | 8910618
 |
Antisense inhibition of basic fibroblast growth factor induces apoptosis in vascular smooth muscle cells.
Fox, J C and Shanley, J R
J. Biol. Chem., 271: 12578-84 (1996)
1996
Mostrar resumen
Basic fibroblast growth factor (bFGF), a potent mitogen for many cell types, is expressed by vascular smooth muscle cells and plays a prominent role in the proliferative response to vascular injury. Basic FGF has also been implicated as a survival factor for a variety of quiescent or terminally differentiated cells. Autocrine mechanisms could potentially mediate both proliferation and cell survival. To probe such autocrine pathways, endogenous bFGF production was inhibited in cultured rat vascular smooth muscle cells by the expression of antisense bFGF RNA. Inhibition of endogenous bFGF production induced apoptosis in these cells independent of proliferation, and apoptosis could be prevented by exogenous bFGF but not serum or epidermal growth factor. The induction of apoptosis was associated with an inappropriate entry into S phase. These data demonstrate that interruption of autocrine bFGF signaling results in apoptosis of vascular smooth muscle cells, and that the mechanism involves disruption of normal cell cycle regulation. | Immunoblotting (Western) | 8647868
 |