239802 Sigma-AldrichCurcumin, Curcuma longa L. - CAS 458-37-7 - Calbiochem
A cell-permeable and irreversible antitumor and anti-inflammatory agent that acts as an inhibitor of 5-lipoxygenase (IC₅₀ = 8 µM) and cyclooxygenase (IC₅₀ = 52 µM).
More>> A cell-permeable and irreversible antitumor and anti-inflammatory agent that acts as an inhibitor of 5-lipoxygenase (IC₅₀ = 8 µM) and cyclooxygenase (IC₅₀ = 52 µM). Less<<Sinónimos: 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, Histone Acetyltransferase Inhibitor I, HAT Inhibitor I, p300/CBP Inhibitor I, NOD2 Signaling Inhibitor I, Nucleotide-binding Oligomerization Domain 2 Signaling Inhibitor I
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| CAS # | Empirical Formula |
|---|---|
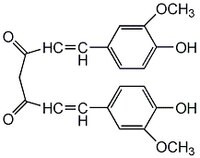
| 458-37-7 | C₂₁H₂₀O₆ |
Products
| Número de referencia | Embalaje | Cant./Env. | |
|---|---|---|---|
| 239802-100MG | Ampolla de plást. | 100 mg |
| Product Information | |
|---|---|
| CAS number | 458-37-7 |
| ATP Competitive | N |
| Form | Orange-yellow solid |
| Hill Formula | C₂₁H₂₀O₆ |
| Chemical formula | C₂₁H₂₀O₆ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | MI5230000 |
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 239802-100MG | 04055977199581 |
Documentation
Curcumin, Curcuma longa L. - CAS 458-37-7 - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
Curcumin, Curcuma longa L. - CAS 458-37-7 - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 239802 |
Referencias bibliográficas
| Visión general referencias |
|---|
| Hung, S., et al. 2008. Mol. Pharmacol. 74, 274. Cui, L., et al. 2007. Antimicrob. Agents Chemother. 51, 488. Salvioli, S., et al. 2007. eCAM 4, 181. Balasubramanyam, K. et al. 2004. J. Biol. Chem. 279, 51163. Brouet, I., and Okshima, H. 1995. Biochem. Biophys. Res. Commun. 206, 533. Korutla, L., and Kumar, R. 1994. Biochim. Biophys. Acta 1224, 597. Flynn, D.L., et al. 1986. Prostagland. Leuk. Med. 22, 357. |
Folleto
| Cargo |
|---|
| Alzheimer's Disease Brochure & Technical Guide |
Citas
| Título | |
|---|---|
|
|